- Laboratory >
- Laboratory medicine >
- Rapid PCR test
Rapid PCR tests
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}

The new SARS-CoV-2 rapid test is a rapid real time PCR test, providing a clear and concise result in a timely manner, enabling patients to take the recommended ...

... the market. The test simultaneously detects 10 bacterial, viral and protozoan infections for a comprehensive sexual health profile. The assay is based on a combination of multiplex PCR and biochip ...
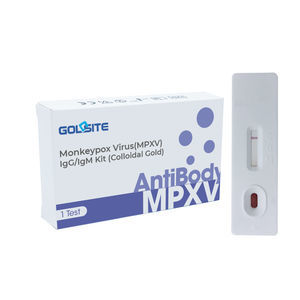
Result display time: 15 min
Lateral Flow Immunoassays Serology Antibody Test Kit for rapid determination of Monkeypox Virus (MPXV) infection history. Product Description is a disease which is attributed to a virus in the same ...

Result display time: 39 min
Specificity: 100 %
Sensitivity: 98 %
... Bosch has developed a new rapid test for its Vivalytic analysis device to detect the SARS-CoV-2 pathogen. The test provides a reliable result in 39 minutes and is currently the fastest ...
Bosch Healthcare Solutions


... fluorescent PCR technique, this kit takes the relatively conserved region of the HIV virus gene coding region as the target region and designs specific primers and fluorescent probes. After the specimen nucleic acid is ...


infectious disease rapid diagnostic testR231T050B0C0
Human Respiratory Virus Detection Kit Prevention and Treatment of Respiratory Infections This kit is intended for the qualitative detection of 18 kinds of respiratory virus, including Rhinovirus (RHV), respiratory ...
Result display time: 30 min
Sensitivity: 99 %
... diagnosis. C.Main Parameter Brand - Bioteke - Model - PR2019 Name - Novel Coronavirus (2019-nCoV, Nucleic Acid Detection Kit (PCR) - Dimension - 60×60×65mm Weight - / - Storage - Cold chain for ...
Bioteke Corporation

Result display time: 10 min
Chemtrue* COVID-19/Influenza A+B Antigen Combo Rapid Test kit (Colloidal Gold) is a rapid test for the qualitative détection of COVID-19 antigen, influenza A antigen ...

Result display time: 60 min
... resistances Laboratory efficiencies with on-demand workflows requiring minimal hands-on time The Impact Fast and accurate PCR testing with Xpert® SA Nasal Complete greatly improves time to result, allowing healthcare ...
Cepheid

Result display time: 60 min
Ring Biotechnology Co Ltd.

... Biosystems 7500 Real-Time PCR Systems Applied Biosystems 7500 Fast Real-Time PCR Systems QuantStudio®5 Real-Time PCR Systems SLAN-96P Real-Time PCR Systems LightCycler®480 ...
Jiangsu Macro micro-test Medical Technology

Result display time: 30 min
Specificity: 96, 95.9 %
Sensitivity: 100 %
... four USB ports. Solana GBS is supported by the power of Virena®. HDA technology Rapid method of isothermal nucleic acid amplification that does not require thermal cycler Lyophilized Master Mix ...
Quidel Eye Health
Result display time: 20 min
Sample volume: 0.03 ml
... symptoms, making it very difficult for patients to receive proper diagnosis and treatment in time. As flu season approaches, rapid and accurate testing has become essential to provide a timely diagnosis and help doctors ...
Credo Diagnostics Biomedical Pte Ltd

Result display time: 15 min
Specificity: 99.3 %
Sensitivity: 93.2 %
... possible. 5.Install the nozzle cap onto the extraction tube tightly. 6.Add 3 drops(around 75μL) into the sample hole of the test card.
Baicare
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group
















Please specify:
Help us improve:
remaining