- Laboratory
- Laboratory medicine
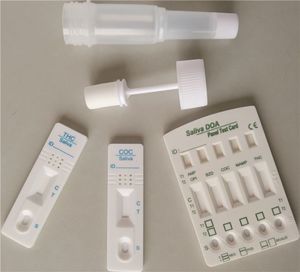
- Rapid drug detection test
- Boson Biotech Co., Ltd.
Rapid drug detection test 1L27P3multi-drugurineimmunochromatographic
Add to favorites
Compare this product
Characteristics
- Application field
- drug detection
- Tested parameter
- multi-drug
- Sample type
- urine
- Analysis mode
- immunochromatographic
- Result display time
5 min
Description
Rapid DOA Test Cup is an immunochromatography based one step in vitro test. It is designed for qualitative determination of drug substances in human urine specimens. This assay may be used in the point of care setting. Below is a list of cut-off concentrations for each drug.
This assay provides only a preliminary analytical test result. A more specific alternative chemical method must be used in order to obtain a confirmed analytical result. Gas chromatography/ mass spectrometry (GC/MS) has been established as the preferred confirmatory method by the Substance Abuse Mental Health Services Administration (SAMHSA). Clinical consideration and professional judgement should be applied to any drug of abuse test result, particularly when preliminary positive results are indicated. The optional built-in Adulteration Test is for validation of urine specimen’s integrity and must not be used for In Vitro diagnostic use.
Catalogs
No catalogs are available for this product.
See all of Boson Biotech Co., Ltd.‘s catalogsOther Boson Biotech Co., Ltd. products
Drugs of Abuse Test Kits
Related Searches
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Cassette rapid diagnostic test
- Virus rapid diagnostic test
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Rapid respiratory infection test
- Urine rapid screening test
- Virus reagent kit
- Test strip
- Bacteria rapid diagnostic test
- COVID-19 rapid diagnostic test
- Strip rapid diagnostic test
- Microbiology reagent kit
- Rapid feces test
- Rapid drug abuse test
- Clinical rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.