- Primary care
- General practice
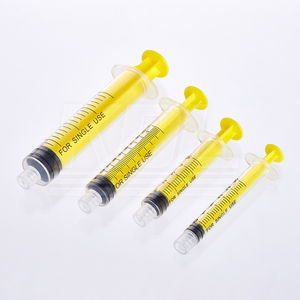
- 20 ml syringe
- Nanchang Kindly Meditech
20 ml syringe 10 ml5 ml50 ml
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Volume
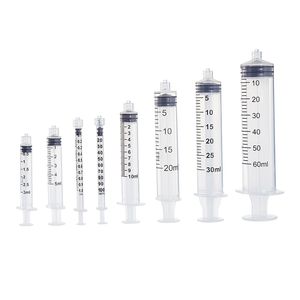
- 10 ml, 1 ml, 5 ml, 0.2 ml, 15 ml, 1.25 ml, 20 ml, 4 ml, 1.5 ml, 1.8 ml, 0.1 ml, 8 ml, 2.2 ml, 350 ml, 3 mL, 1000 mL, 2 ml, 12 ml, 50 ml, 60 mL, 250 mL, 30 ml, 40 ml, 0.85 mL, 500 mL, 0.25 ml, 100 ml, 0.3 mL, 7 ml, 2.5 ml, 3000 ml, 11 ml, 125 ml, 6 ml, 200 ml, 0.6 mL, 25 ml, 115 ml, 65 ml, 300 mL, 22 ml
- Material
- polypropylene, rubber, latex-free
- Options
- disposable, sterile, luer lock, 3-piece, 2-piece, slip tip
Description
- Medical grade raw materials, Sterile, non-toxic, non-pyrogenic
- Transparent barrel
- Material for gasket: IR rubber, Latex free
- Standard: ISO7886-1
- Multiple type cap
- Manufactured in accordance with ISO 13485
Product Features:
- Intended use: Sterile syringes are intended to inject drug for patients.
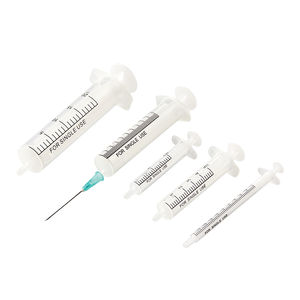
- Structure and composition: Protective cap, Barrel, Plunger stopper, Plunger.
- Main Material: PP, IR rubber, Silicone Oil
- Shelf life: 5 years
- Certification and Quality Assurance: In compliance with Medical Regulation (EU) 2017/745(Class Ims)
- Manufacturing process is in compliance with ISO 13485 Quality System
Product Parameters:
- Variant 1: Three parts, without needle, luer slip(centre), latex free
Syringes variants: 1, 2, 2.5, 3, 5, 10, 20, 50, 60ml
- Variant 2: Three parts, without needle, luer slip(centre), latex free
Syringes variants: 10, 20, 25, 30, 35, 50, 60ml
- Variant 3: Three parts, without needle, luer slip(centre), latex free
Syringes variants: 1, 2, 2.5, 3, 5, 10, 20, 30, 35, 50, 60, 100ml
- Variant 4: Two parts, without needle, luer slip(centre), Latex free
Syringes variants: 1ml
- Variant 5: Two parts, without needle, luer lock, Latex free
Syringes variants: 1ml
- Variant 6: Three parts, without needle, luer slip(centre), latex free
Syringes variants: 1, 2, 2.5, 3, 5, 10, 20, 50, 60ml
- Medical grade raw materials
- Sterile, non-toxic, non-pyrogenic
- Transparent barrel
- Gasket material: IR rubber, latex free
- Standard: ISO7886-1
- Multiple type cap
- Manufactured in accordance with ISO 13485
- Intended use: Drug injection for patients
- Structure: Protective cap, barrel, plunger stopper, plunger
- Main materials: PP, IR rubber, silicone oil
- Shelf life: 5 years
- Certification: Medical Regulation (EU) 2017/745 (Class Ims), ISO 13485
Catalogs
No catalogs are available for this product.
See all of Nanchang Kindly Meditech‘s catalogsExhibitions
Meet this supplier at the following exhibition(s):

China International Medical Equipment Fair
26-29 Sep 2025 guangzhou (China) Hall 5.2 - Stand H-E23
More information

Other Nanchang Kindly Meditech products
Drug Delivery Devices
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.