- Secondary care
- Otorhinolaryngology
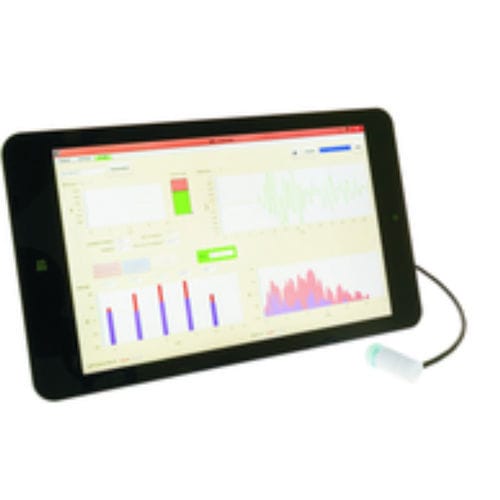
- OAE screening system
- Pilot Blankenfelde
OAE screening system CORONA E3for pediatric audiometrytablet-based
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Type of system
- OAE screening system
- Type of patient
- for pediatric audiometry
- Technology
- tablet-based
Description
In audiology the measurement of Otoacoustic Emissions are an important component for testing the function of the cochlea. It is also a globally recognized measurement method for use in newborn hearing screening. However, the reliability of the diagnosis and the speed of the measurement are highly dependent on the environmental noise floor. Often in newborn stations and in incubators such measurements cannot be performed due to high ambient noise. The reason is a too tiny signal-to-noise ratio (SNR) which does lead into a low specificity of the measurement.
The new development Active-Noise-Reduction system records also the background noise allowing the user a direct and individual adaption of the OAE measurement to the environmental noise.
Advantages of the method compared with conventional OAE - methods:
Highly reduced influence of the ambient noise
High reproducibility of the measurement results
Faster measurement due to low artifact rates
Catalogs
No catalogs are available for this product.
See all of Pilot Blankenfelde‘s catalogsRelated Searches
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.


