- Secondary care
- Orthopedic surgery
- Access sheath spine augmentation system
- Synimed Synergie Ingénierie Médicale

- Products
- Catalogs
- News & Trends
- Exhibitions
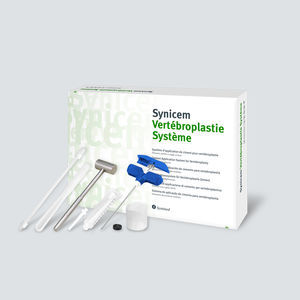
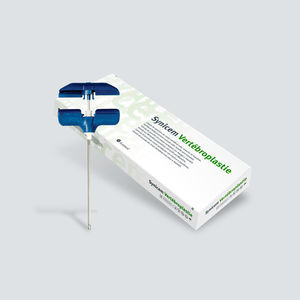
Access sheath spine augmentation system percutaneous vertebroplasty
Add to favorites
Compare this product
Characteristics
- Device type
- access sheath
- Applications
- percutaneous vertebroplasty
Description
Each carton contains one ETO sterilized blister with one of the following cannulas:
External diameter: 2.4 mm / Length: 100 mm
External diameter: 3.0 mm / Length: 100 mm / 150 mm.
Components:
• Surgical grade stainless steel cannula with metal handle.
• Surgical grade stainless steel mandrel.
Catalogs
No catalogs are available for this product.
See all of Synimed Synergie Ingénierie Médicale‘s catalogsExhibitions
Meet this supplier at the following exhibition(s):

Other Synimed Synergie Ingénierie Médicale products
VERTEBROPLASTY
Related Searches
- Agitator
- Orthopedic surgery surgery set
- Blender
- Sterile surgical set
- Single-use surgical set
- Bone cement
- Vacuum blender
- Orthopedic surgery bone cement
- Spine augmentation system
- Temporary spacer
- Cranial implant
- Custom-made cranial implant
- Percutaneous vertebroplasty spine augmentation system
- Bone cement mixer
- Femoral fracture surgery instrument kit
- Temporary hip spacer
- Access sheath spine augmentation system
- Mixer without display
- Portable blender
- Orthopedic surgery mixer
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.