- Surgery unit
- Surgical Instruments
- Absorbable suture thread
- Vitrex Medical A/S
- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
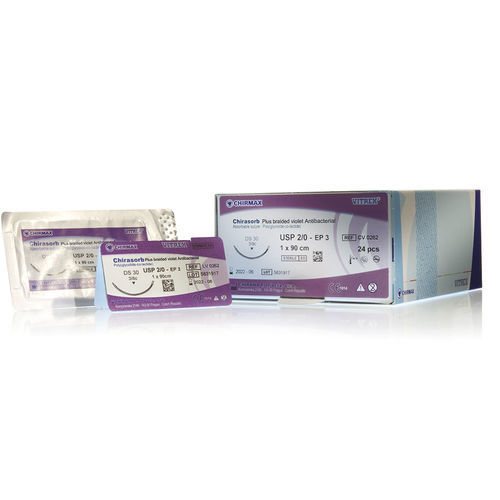
Mid-term absorbable suture thread Chirasorb Plussoft tissue surgery5-04-0
Add to favorites
Compare this product
Characteristics
- Resorbability
- mid-term absorbable
- Use
- soft tissue surgery
- Thread diameter (USP)
- 2, 3-0, 4-0, 2-0, 0, 5-0, 1
- Number per box
20 unit
Description
Chirasorb Plus braided is a mid-term absorbable, antibacterial coated, synthetic, multifilament suture made of 90% glycolide and 10% L-lactide. The braided suture is covered with antibacterial coating, which consists of copolymer glycolide and lactide, calcium stearate and chlorhexidine diacetate.
Chlorhexidine is a cationic antiseptic with a wide range of antimicrobial effects. It is effective on both Gram-positive and Gramnegative bacteria; the mechanism of action is cytoplasmic membrane disruption.
Sterile Chirasorb Plus braided is available in violet or natural. The product elicits a minimal acute inflammatory reaction in tissues.
Progressive loss of tensile strength and absorption of Chirasorb Plus braided occurs by hydrolysis, when the polymer is degraded to glycolic and lactic acids, which are subsequently absorbed and metabolised by the body.
Indication:
General soft tissue approximation and/or ligation in all specialties.
Contraindication:
Chirasorb Plus braided should not be used in tissues subject to expansion, stretching or distension, or which might require long-term mechanical support. Chirasorb Plus braided should not be used in patients with known allergic reactions to Chlorhexidine.
Structure:
Multifilament
Absorption method:
Hydrolysis
Strength Retention Profile:
MID-TERM HEALING (35 days)
1 Day - 100%
14 Days - 75%
21 Days - 50%
Mass Absorption profile:
56 – 70 days
Catalogs
Exhibitions
Meet this supplier at the following exhibition(s):

Related Searches
- Vitrex suture thread
- Vitrex non-absorbable suture thread
- Vitrex general surgery suture thread
- Vitrex absorbable suture thread
- Veterinary needle
- Orthopedic surgery suture thread
- Vitrex 2 suture thread
- Ophthalmic surgery suture thread
- Vitrex 2-0 suture thread
- Vitrex 0 suture thread
- Vitrex 3-0 suture thread
- Vitrex 4-0 suture thread
- Vitrex 6-0 suture thread
- Vitrex 5-0 suture thread
- Cardiovascular surgery suture thread
- Vitrex plastic surgery suture thread
- Vitrex 1 suture thread
- 5 suture thread
- Oral surgery suture thread
- 7-0 suture thread
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.