Website:
PhysioFlow
Website:
PhysioFlow
Group: Manatec Biomedical
PhysioFlow
About Us
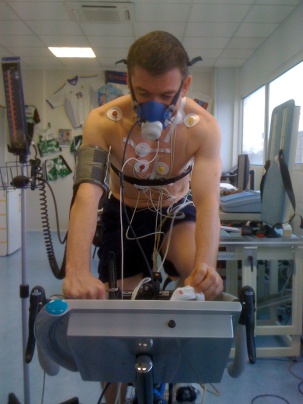
PhysioFlow® is the only cardiac output monitor that can measure almost patients in very demanding situations, ranging from intensive care in shocked patients to maximal exercise in top athletes. Also, its signals, parameters, displays, ease of use, non invasiveness and limited cost per patient makes it the ideal tool to perform screening of many cardiovascular abnormalites, and this in a sensitive manner (early detection of pathologies).
The research work was initiated by a French team in the mid 80's. The team was led by Jean Bour, MD who was head of a cardiology, internal medicine and critical care unit of a general hospital. His concern was to develop a non invasive method to measure hemodynamic parameters at rest and during exercise. His studies are based on thoracic electrical bioimpedance concepts, but with an enhanced methodology.
At first the approach was merely clinical and empirical. The group collected an extensive patient database, with widely different physio-pathological and hemodynamic parameters, and varying impedance signal patterns. By using this database, their idea was to categorize signal patterns and find a model based on pure signal morphology analysis, avoiding the need to measure baseline impedance (Z0).
Dr. Bour first patented the system in France in 1993. Validation studies were performed later in the French University Hospitals of Strasbourg, Rennes, and Angers, among others. Manatec Biomedical was incorporated in 1995 and started operations in June 1996 in France. The company started to manufacture and market a first series of devices, called PhysioFlow® PF-03™. These devices derived directly from the last impedance cardiograph prototypes developed by Dr. Bour.
After that experience, we started to market PhysioFlow® PF05 Lab1™ in 1999, with improved new features and user friendliness. The software was entirely redesigned under Windows™ 95/98, and CE marking obtained. The PhysioFlow technology is approved in many countries, including Europe, the USA, China, Japan, Australia, etc...)? It has a market presence in over 40 countires.
A sister company PhysioFlow® Inc. was incorporated in the USA in 2006. The group entered a strategic partnership with Vasocom Inc. (now Neumedx Inc.) in order to market the technologies in North America. US FDA approval was obtained for PhysioFlow® PF05 Lab1™ in April 2008 and the new portable device PhysioFlow® PF07 Enduro™ was launched in December 2010, followed by the Q-Link model.
PhysioFlow® is now established as a standard of care in resting and exercise hemodynamics and recommended for clinical use by the A.H.A, the E.S.C and in China. 1200+ units have been sold in 50+ countries

Our values
The PhysioFlow® group is committed to providing the best possible products and services to its customers and to the patients worldwide. The mission of the entire team is to establish PhysioFlow® as a standard of care in cardiology and lung disease. PhysioFlow® may improve the quality of care delivered to patients, the practice of medical professionals and the cost effectiveness of healthcare.
In spite of a fiercely competitive environment the company has decided to comply with higher ethical standards that supersede the industry's common practice. Below are some examples of these ethical standard:
In order to support our promotional efforts we try to only quote scientists, physicians, or studies that actually use our devices and not competitive systems. If they do not use our device we will mention it.
Besides the co-inventor and co-founder, Dr. Jean Bour, no other physician owns interest in our companies. In particular we have not offered stock-options or consulting fees to physicians promoting our technology for instance during scientific conventions.
We strive to claim only features that are supported by scientific studies published by strictly independent teams.
We avoid making false or unsubstantiated claims like this one by one of our competitors: "The Only Noninvasive Cardiac Output Monitor Whose FDA Predicate is The Swan Ganz" (PhysioFlow®, like some other noninvasive hemodynamic monitors, has used the Swan Ganz as a predicate for FDA approval purposes as well).
In the sales process the company will always do its best to focus on customers and patient's interests and not seek a short-term financial advantage at any cost.