- Laboratory >
- Laboratory medicine >
- Nasopharyngeal test kit
Nasopharyngeal test kits

... presentation and the results of other laboratory tests. Results from the Amazing COVID-19 Ag/Ab(IgG/IgM) and Influenza Ag(A/B) Sealing Tube Twin Test Strip should not be used as the sole basis for ...
Amazing Biotech Co., Ltd

Result display time: 15 min
This kit is used to the qualitative detection of SARS-CoV-2 antigen presents in humanoropharyngeal swab and nasopharyngeal swab. Value-added Function: Ø Colloidal gold labelling technology, no device ...
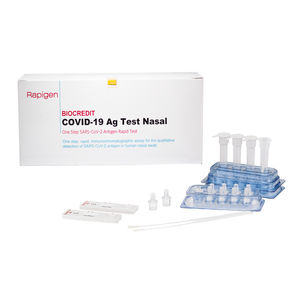
Result display time: 10 min - 15 min
Specificity: 100 %
Sensitivity: 98.1, 93.1 %
... symptoms. It provides only an initial screening test result and more specific alternative diagnosis methods should be performed in order to confirm COVID-19 infection. Principle of Test BIOCREDIT ...
Biz Distribution
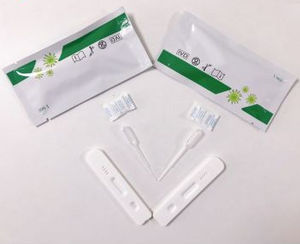
Categories Testing and vaccines Brand gyf Model COVID-19-antigen home self-test kit specification 50pcs/box Certification ce Certification fda Certification cfda accuracy 98% Storage method ...
Tianjin Guyufan Biological Technology

Result display time: 15 min
The Influenza A+B Test detects and differentiates influenza type A and type B antigens directly from nasal swab and nasopharyngeal swab specimens. A single sample can be used to run both the Influenza ...
... N501Y SARS-nCoV-19 -qRT-PCR Detection Kit is an in vitro diagnostic medical device that is used for qualitative detection of SARS-CoV-2 Variant of Concern 202012/01 (VOC-202012/01) assumed to emerged ...

Result display time: 75 min - 80 min
Sensitivity: 96.4 % - 99.5 %
... Registration certificate: CE:Ref. No.:GZ 8821-2020; China NMPA: GUOXIEZHUZHUN 20203400229 Specification: 48 tests/kit;96 tests/kit Amplification time: 1h20min Instrument ...
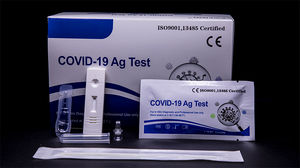
Result display time: 10 min - 15 min
Specificity: 99.6 %
Sensitivity: 98.1 %
COVID-19 Ag Test is used for qualitative detection of the COVID-19 antigen in human nasopharyngeal or pharyngeal swab specimens.

... Extraction Vial five times just before applying the sample to the Test Strip. 7.Dispense 80µL (3 drops) of the specimen into the sample well on the cassette, and start the timer. 8.Interpret the test ...
Result display time: 10 min
1 × 5 Bags, each bag contains 1 test cassette and 1 desiccant 1 × 5 Extraction tube 1 × 5 Extraction tube nozzle 1 × 5 Sample Extraction Tube Bottle 1 × 5 Individually packaged sample for sampling the sterile swab 1 ...

Real-time PCR Kit for Rapid Detection of Novel coronavirus (SARS-CoV-2) 【Detection Principle】 This kit is based on one-step RT-qPCR technique, Novel Coronavirus (SRAS-COV-2) ORF1ab and N gene are ...
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group














Please specify:
Help us improve:
remaining