- Secondary care
- Urology
- Urinary incontinence reconstruction mesh
- AMI - Agency for Medical Innovations
Urinary incontinence reconstruction mesh PFR5021retropubic approachtransobturator approachwomen
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Mesh type
- urinary incontinence
- Surgical technique
- transobturator approach, retropubic approach
- Patient type
- women
Description
Treatment of Stress Urinary Incontinence
Indication: Surgical treatment of female stress urinary incontinence resulting from
urethral hypermobility and/or
intrinsic sphincter deficiency (ISD)
after failed conservative treatment methods.
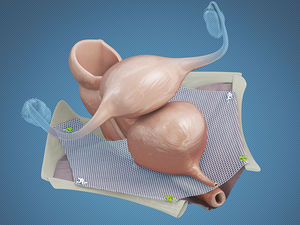
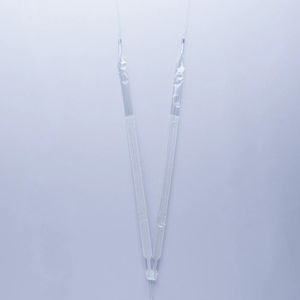
Mid-urethral sling implant
The A.M.I. Multi Purpose Sling is a midurethral implant made of polypropylene mesh, pull-in sutures and a detachable pull-in aid.
Implanted to support the female bladder neck.
The sling’s center is intended to be positioned midurethral, while the lateral sling arms enable the fixation of the sling in the tissue.
Features
Firm, macroporous, biocompatible, monofilament, polypropylene mesh.
Protective cover to facilitate the placement of the sling.
Benefits
Efficient improvement of incontinence in female patients with SUI.
Very low rate of intra-operative complications (<0.5%).
Very low rate of post-operative complications in a 3-month follow-up period (<1%).
Functioning principles of A.M.I. Multi Purpose Sling
Supports female bladder neck
Slings are implanted to support female bladder neck
Sling’s center is intended to be positioned midurethral, while lateral sling arms enable fixation of the sling in the tissue
Implantation via retropubic or transobturator approach possible
Combinable products
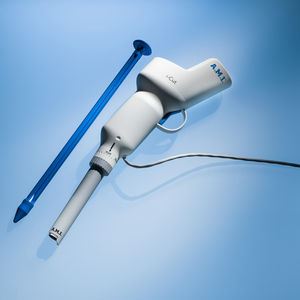
Tunnellers
Multiple use devices, which are designed to meet the anatomic requirements for implant insertion.
A slot at their tip enables the attachment of the sling pull sutures, which are used to pull the implant in place.
Tunneller for transobturatoric approach (outside-in / inside-out)
Tunneller for retropubic approach
Catalogs
Solutions for Urogynecology
36 Pages
Other AMI - Agency for Medical Innovations products
Urogynaecology
Related Searches
- Reconstruction mesh
- Women reconstruction mesh
- Urinary incontinence reconstruction mesh
- Prolapse reconstruction mesh
- Transobturator approach reconstruction mesh
- Vaginal approach reconstruction mesh
- Morcellator
- Cystocele reconstruction mesh
- Laparoscopic approach reconstruction mesh
- Uterine morcellator
- Colpocele reconstruction mesh
- Rectocele reconstruction mesh
- Retropubic approach reconstruction mesh
- Sphincter prosthesis
- Urinary incontinence sphincter prosthesis
- Vesical sphincter prosthesis
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.