- Medical Technical Facilities
- Intensive care
- Single blood bag
- Bionic Medizintechnik
- Products
- Catalogs
- News & Trends
- Exhibitions
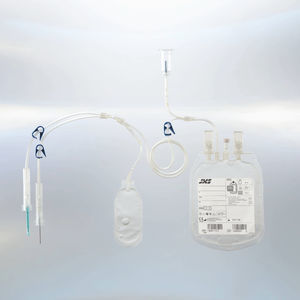
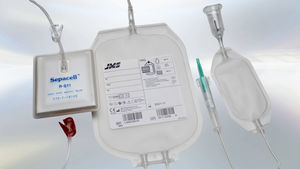
Double blood bag R-S11singletriplequadruple
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Type
- double, single, triple, quadruple
- Anticoagulant
- CPD/SAG-M, CPDA-1
- Function
- for platelets, for red blood cells, for blood plasma
- Other characteristics
- single use
Description
JMS Blood Bags are designed to ensure the highest quality in blood collection, separation, preservation, and transfusion. Manufactured under strict quality control, they offer a safe and reliable solution for both donors and users. With an integrated production process that spans from raw materials to the final product, JMS maintains the highest standards of quality and safety. The blood bags are available in various configurations, catering to the diverse needs of blood banks and healthcare providers worldwide.
- Highest Quality and Safety
- Flexible Configurations
- Integrated In-House Production
- Extended Shelf Life
Each bag is equipped with a high-quality needle that ensures optimal sharpness and precise puncture characteristics. To further enhance safety, a needle protector minimizes the risk of accidental needle-stick injuries. Additionally, the blood bags feature an extra port cover to prevent direct contact, ensuring a sterile environment throughout the process. The double packaging system, consisting of an aluminum foil and blister pack, extends the shelf life of the product to 36 months, allowing for greater flexibility in storage and use.
The process of blood component separation follows a structured procedure to ensure efficiency and safety. Whole blood is first collected in the primary bag and then subjected to centrifugation, allowing for the separation of red blood cells, platelet-rich plasma, and buffy coat. These components are then transferred into designated satellite bags for further processing or storage, making them readily available for transfusion or long-term preservation.
JMS offers a comprehensive selection of blood bags tailored for different applications. Single blood bags are ideal for basic collection and storage, while double, triple, and quadruple blood bags allow for the separation of multiple blood components, including red blood cells, plasma, buffy coat, and leukocytes. The Top & Bottom systems facilitate precise blood component separation and are compatible with most automatic extractors. Additionally, the inclusion of CPDA-1 and CPD-SAGM solutions enhances blood preservation and storage, ensuring optimal performance.
With over 30 years of experience in this field, JMS maintains the highest standards in blood bag production. All products are manufactured under clean room conditions and undergo strict sterilisation procedures to ensure safety and reliability. JMS blood bags comply with international regulations and standards, making them a reliable choice for healthcare facilities around the world.
- Product highlights: Highest quality and safety, flexible configurations, integrated in-house production, extended shelf life
- Advanced design: High-quality needle, needle protector, extra port cover, double packaging (aluminum foil and blister pack), 36 months shelf life
- Blood management: Structured separation process, satellite bags for components, suitable for transfusion and preservation
- Product range: Single, double, triple, quadruple bags; Top & Bottom systems; CPDA-1 and CPD-SAGM solutions
- Manufacturing: Over 30 years experience, clean room production, strict sterilisation, international compliance
Catalogs
No catalogs are available for this product.
See all of Bionic Medizintechnik‘s catalogsRelated Searches
- Dialysis chair
- Sterile filter
- Line filter
- Blood filter
- Blood bag
- Leukoreduction filter
- CPDA-1 blood bag
- Bloodline
- Hemodialysis bloodline
- CPD blood bag
- Single blood bag
- Quadruple blood bag
- Single use blood bag
- Double blood bag
- Red blood cells filter
- Triple blood bag
- Blood plasma blood bag
- Platelet blood bag
- Red blood cell blood bag
- Process filter
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.