- Secondary care
- Cardiology
- PTA catheter
- Biosensors International
PTA catheter BIOPATH™ 035peripheralfemoral arteryballoon
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Application
- PTA
- Area of the body
- peripheral, femoral artery
- Options
- balloon, drug eluting, hydrophilic
- Total length
80 cm, 135 cm
(31 in, 53 in)- Balloon diameter
4 mm, 5 mm, 6 mm, 7 mm, 8 mm
(0.16 in, 0.2 in, 0.24 in, 0.28 in, 0.31 in)
Description
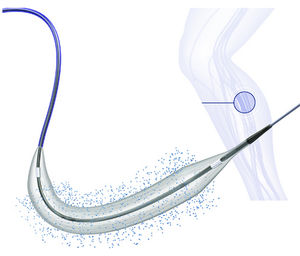
Designed for superficial femoral artery interventions
BioPath™ 035 is a drug-eluting balloon dilatation catheter designed for percutaneous transluminal angioplasty (PTA) and has been optimized for the treatment of patients with peripheral arterial disease.
BioPath™ 035 is intended for peripheral interventions above the knee. This paclitaxel-eleluting balloon feature a proprietary shellac coating technology which consistently delivers paclitaxel, an anti restenotic drug during very brief inflation times, while also minimizing washout of the drug during delivery and placement of the drug-eluting balloon.
BioPath™ 035 balloon catheter offer excellent pushability, trackability and crossability due to a low balloon profile, low tip entry profile and hydrophilic coating on the distal shaft of the catheter.
The right reach
The treatment process
With balloon dilatation, the injuries to the arterial wall initiate an inflammatory reaction with an excretion of growth factors which trigger the onset of cell division and smooth muscle cell migration.
Paclitaxel prevents restenosis by stabilizing microtubal formation and thus prevents the cells going through the phases of replication, resulting in the inhibition of cell division.
Paclitaxel reduces the excretion of the platelet derived growth factor (PDGF) that mediates vascular smooth muscle cell migration to the intima.
The BioPath™ 035 balloon coating
The BioPath 035 balloon coating consists of a 1:1 mixture of paclitaxel (3 μg/mm2) and shellac, a natural resin approved by the FDA (GRAS), and by Europe (E904) as a food additive.
Catalogs
BioPath 035
2 Pages
Related Searches
- Catheter
- Balloon catheter
- Stent
- Dilatation catheter
- Peripheral catheter
- Cardiac catheter
- Metal stent
- Coronary catheter
- Hydrophilic catheter
- PTCA catheter
- Coronary stent
- PTA catheter
- Cobalt-chromium stent
- Coated stent
- Drug eluting stent
- Arterial catheter
- Valve bioprosthesis
- Drug eluting catheter
- Bovine tissue valve bioprosthesis
- Aortic valve bioprosthesis
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.