- Products
- Catalogs
- News & Trends
- Exhibitions
Analysis software Dr. Noon CKDdisease risk managementmedicalfor nephrology
Add to favorites
Compare this product
Characteristics
- Function
- analysis, disease risk management
- Applications
- medical, for nephrology
- Area of the body
- kidney
- Type
- artificial intelligence
Description
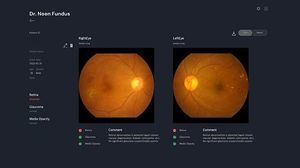
Retina-based AI solution that predicts the future risk of chronic kidney disease risk.
Product Details:
- Regulation Info:
- Dr. Noon CKD_Korea MFDS: [Product Name] DrNoon CKD / Approved for clinical trial protocol (Jan. 2024) and designated as a Breakthrough Device (Aug. 2023) by the Ministry of Food and Drug Safety of Korea
*Dr. Noon CKD is under the approval process by MFDS of Korea
AI-powered Solution for Chronic Kidney Disease Risk Assessment
Dr. Noon CKD is a retina-based AI solution that predicts the future risk of developing chronic kidney disease. Dr. Noon CKD analyzes the blood vessels in the retina with a simple retinal scan without the need for blood or urine tests and shows the risk of developing chronic kidney disease.
How Dr. Noon CKD works:
- STEP 1: Eye Scan
Take one picture of each eye to capture the retina.
- STEP 2: Image Upload
The retinal images are uploaded to the healthcare institution’s server or cloud platform.
- STEP 3: AI Analysis
Artificial intelligence automatically analyzes the images to assess the patient’s risk of chronic kidney disease.
- STEP 4: Result Review
The risk of chronic kidney disease is determined through an automatically generated report.
Comparison with Traditional Methods
A comparison with traditional methods highlights Dr. Noon CKD's superior performance over conventional tests in assessing cardiovascular risk.
- Blood Test (eGFR)
- Urine Test (ACR)
Key Benefits
- High Accuracy: Provides higher predictive accuracy for chronic kidney disease risk than estimated GFR (eGFR), the current standard kidney function test.
- Safe & Non-Invasive: Requires no needle injection or radiation exposure, making it comfortable for patients.
- Fast Test Results: Results are delivered promptly, allowing patients to receive their reports without delay.
- High Accessibility: It is designed for use in diverse medical settings such as clinics, hospitals, and screening centers, just like standard blood tests.
Publications & Webinars
- Non-invasive chronic kidney disease risk stratification tool derived from retina-based deep learning and clinical factors
- Prediction of systemic biomarkers from retinal photographs: development and validation of deep-learning algorithms
- Retinal Photograph-based Deep Learning Predicts CKD among people with preserved kidney function
Technical Specifications / Features
- AI software medical device
- Predicts future risk of chronic kidney disease using retinal images
- Non-invasive, no blood or urine test required
- Designed for clinical workflow integration
- Requires only a fundus camera
- Regulatory status: Approved for clinical trial protocol (Korea, Jan. 2024), Breakthrough Device designation (Korea, Aug. 2023)
Exhibitions
Meet this supplier at the following exhibition(s):

*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.