- Laboratory >
- Laboratory medicine >
- Flu test kit
Flu test kits
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}

Result display time: 75 min
Real-time RT-PCR test for the qualitative detection of HA gene from Influenza A/B virus and N and ORF1a gene from SARS-CoV-2 in nasopharyngeal swab. Details Features: Hot Start PCR: high specificity Detection ...
Result display time: 10 min
... as highly contagious respiratory disease but triggered by different virus: SARS-CoV-2 and Flu A&B, respectively. GOLDSITE SARS-CoV-2 & Influenza A/B Antigen Kit (Colloidal Gold) helps healthcare professionals ...
Result display time: 15 min
... The COVID-19, Flu, RSV combined test is an option if you would like to determine what type of infection you have: SARS-CoV-2, influenza A/B, and/or respiratory syncytial virus (RSV).GOLDSITE SARS-CoV-2 ...
Result display time: 15 min
... develop a serious flu complications. There are two main types of influenza (flu) viruses: Types A and B. The influenza A and B viruses that are widespread among people are to blame on seasonal flu ...

Result display time: 3 h
... testing. With Panther® Scalable Solutions, you can run over 1000 tests in 24 hours, attaining first results in about 3 hours. The Panther Fusion® SARS-CoV-2 assay is a real-time PCR test and the Aptima® ...
Result display time: 15 min
FLU A/B Antigen Test Kit (Colloidal Gold) is used for in vitro qualitative detection of influenza A/B antigen in human nasopharyngeal swab samples. PACK FORMATS 1 Test/Box 10 ...
Result display time: 15 min
Sample volume: 0.1 ml
Specificity: 96.7 % - 99.7 %
COVID-19 & FLU A/B Antigen Test Kit (Colloidal Gold) is used for in vitro qualitative detection of 2019 Novel Coronavirus antigen and influenza A/B antigen in human nasopharyngeal swab ...
SCREEN TEST COVID-19+FLU A/B Sars-Cov-2 Antigen + Flu A/B Rapid Test REF: SC-1262-20 The Covid-19 Antigen Rapid Swab and Influenza A + B (nasopharyngeal swab) ...
... vitro diagnostic use. The RIDA®GENE Flu & SARS-CoV-2 test, performed on the LightCycler® 480II real-time PCR instrument, is a multiplex real-time RT-PCR for the direct qualitative detection and differentiation ...
For in vitro diagnostic use. The RIDA®GENE Flu & RSV test, performed on the Roche LightCycler® 480II, is a multiplex real-time RT-PCR for the direct qualitative detection and differentiation of influenza ...
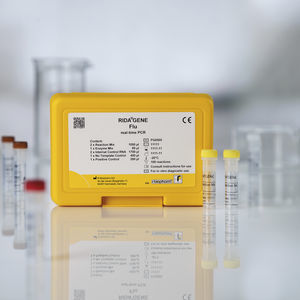
For in vitro diagnostic use. The RIDA®GENE Flu test, performed on the Roche LightCycler® 480II, is a multiplex real-time RT-PCR for the direct qualitative detection and differentiation of influenza virus ...

Result display time: 15 min
Specificity: 95, 96, 94.6, 97 %
Sensitivity: 90, 97.1, 89 %
... the FDA authorizes tests for use at the point of care (including SARS-CoV-2 point of care test systems) under an EUA, such tests are deemed to be CLIA waived tests. Accordingly, ...
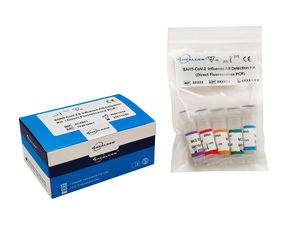
... Influenza A/B Detection Kit (Direct Fluorescence PCR) Sample Type: Oropharyngeal swab or Nasopharyngeal swab Assay Target : SARS-CoV-2, Flu A, Flu B Analytical Sensitivity(LoD): ...
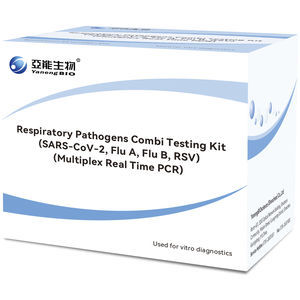
Several epidemiologists and CDC officials recommended the Respiratory Pathogens Multiplex PCR test for flu season. Positive results from this kits are indicative of the presence of SARS-CoV-2, ...

Result display time: 90 min
Sample volume: 0.008, 0.02 ml
... 19 & Flu A/B Multiplex One-Step RT-qPCR Kit, IVD is built to have the broadest detection profile possible whilst remaining specific to the SARS-CoV-2 and Influenza types A and B genomes. NZYTech’s ...
NZYTech

... only. For professional use only. TEST PRINCIPLE This kit uses double antibody sandwich method and fluorescence immunochromatography analysis technology to quantitatively detect the concentration ...

... identification of major respiratory viruses (Flu A, Flu B, RSV, MPV, AdV, HRV, PIV and SARS-CoV-2) allows attentive patient care and effective infection management. KEY FEATURES AND BENEFITS Syndromic ...

300+ items; RT-PCR; SARS-CoV-2; Variant; Omicron; Flu A, Flu B; RSV; Tuberculosis; Dengue; Respiratory Pathogens Multiple Detection Kit A variety of detection ...
Result display time: 15 min
... nasopharyngeal swab or oropharyngeal swab related to the respiratory infection, including antigens of Influenza A (Flu A), Influenza B (Flu B), Respiratory syncytial virus (RSV), human Adenovirus (hAdV) ...

... influenza is often the first virus people think of.3 In the US, the CDC estimates that 8% of the population will be sick from the flu each year, varying from 3-11% depending on the year.4 Some viruses, such as Respiratory ...

This kit is designed for the identification of Influenza A virus, Influenza B virus, and 2019-nCoV in respiratory samples. With high specificity, sensitivity and repeatability, this kit is able to assist ...
Respiratory-Borne Pathogen Detection Kit Respiratory infection refers to a disease of infectivity due to the invasion and infection of pathogens into respiratory tract via the nasal cavity, throat, ...
2019-Novel Coronavirus, Influenza A and B Virus Nucleic Acid Test Kit This kit is intended for the qualitative detection of ORF1ab gene of 2019-Novel Coronavirus (2019-nCoV, SARS-COV-2), ...
Jiangsu Mole Bioscience

Result display time: 45 min
... Pandemic LC kit is intended for the detection of Influenza A and B RNA together with Pandemic variant A/H1N1. Principle of the method This diagnostic kit is based on Reverse Transcription-quantitative ...

Result display time: 120 min
GeneFinder™ COVID-19/Flu A&B RealAmp Kit is the One-Step Reverse Transcription Real-Time PCR Kit designed to detect both Novel Corona virus (SARS-CoV-2, COVID-19) and Influenza A&B virus ...
Result display time: 30 min
Specificity: 100 %
Sensitivity: 100 %
Influenza viruses are categorized in type A, B, and C on the basis of the antigenic differences of their nucleoprotein (NP) and matrix (M1) proteins. Only type A and B are perceived to be clinically relevant in humans. Influenza virus ...

Regulatory Status (USA):510(k) Exempt Discipline:Infectious Disease

Result display time: 15 min
... minute, self-test is waiting at home for when you get back. Just $8.99 per test, so you can load up your first aid kit or travel bag. Each iHealth COVID-19 Antigen Rapid Test ...

Result display time: 30 min - 70 min
Specificity: 95 %
Sensitivity: 95 %
... Product Information FlashDetect™ Influenza A Virus and Influenza B Virus Detection Kit (PCR- Fluorescence Probe) is a real-time RT-PCR test intended for the qualitative detection of ...

Result display time: 40 min
... detection, enabling the simultaneous detection of all significant respiratory viral targets. SMARTCHEK® SARS-CoV-2/Flu/RSV Detection Kit is a reverse transcription real-time polymerase ...
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group


























Please specify:
Help us improve:
remaining