- Laboratory >
- Laboratory medicine >
- Pneumonia test kit
Pneumonia test kits
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}

... 2) Serological methods; 3) Nucleic acid amplification test. The kit developed by Tianlong is suitable for the auxiliary diagnosis of mycoplasma pneumoniae. The test results of ...

... 2) Serological methods; 3) Nucleic acid amplification test. The kit developed by Tianlong is suitable for the auxiliary diagnosis of mycoplasma pneumoniae. The test results of ...

Intended use: The TianLong Respiratory 7 types Pathogen Multiplex Nucleic Acid Detection Kit is a multiplexed nucleic acid test intended for use with Panall 8000 MD System for the ...

Result display time: 10 min
... cause the transmission of canine infectious diseases (canine distemper), and cause clinical symptoms such as conjunctivitis, pneumonia and gastroenteritis in dogs. High mortality, strong infectivity and short course of ...

Result display time: 10 min
Sample volume: 0.0025 ml
This product uses fluorescence immunochromatography to quantitatively detect CDV IgG antibody content in dog blood. Basic principle: there are T and C lines on the nitric acid fiber membrane. Combination mat can spray has specific recognition ...

Result display time: 10 min
Sample volume: 0.01 ml
... nose, and the secretions begin to be serous. As the disease worsens, it becomes purulent. Some sick cats have oral ulcers, pneumonia and vaginitis, and some skin ulcers. The disease is a serious hazard to kittens, and ...
Result display time: 5 min
... in vitro quantitative determination of Krebs von den Lungen 6 in human serum, plasma. The test kit is used as an aid in the diagnosis of interstitial pneumonia disease activity. For ...

pneumonia detection kitcareGENE™ Pneumonia-12 Detection
... USE In vitro diagnostic medical device for diagnosing Pneumonia careGENE™ Pneumonia detection kit is a product that can qualitatively detect pneumonia-causing ...
... USE In vitro diagnostic medical device for diagnosing Pneumonia careGENE™ Pneumonia detection kit is a product that can qualitatively detect pneumonia-causing ...
For in vitro diagnostic use. The RIDA®GENE Flu & SARS-CoV-2 test, performed on the LightCycler® 480II real-time PCR instrument, is a multiplex real-time RT-PCR for the direct qualitative detection and differentiation ...
R-Biopharm AG

For in vitro diagnostic use. The RIDA®GENE SARS-CoV-2 test, which will be performed on the Roche LightCycler® 480II, is a multiplex real-time RT-PCR for the direct qualitative detection of coronavirus (SARS-CoV-2) RNA ...
R-Biopharm AG
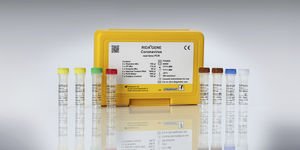
For in vitro diagnostic use. The RIDA®GENE Coronavirus Test performed on the LightCycler® 480 II real-time PCR instrument is a multiplex real-time RT-PCR for the direct qualitative detection and differentiation of coronaviruses ...
R-Biopharm AG
Sample volume: 0.05, 0.025 ml
... these inflammatory markers are often used in the fields of atherosclerosis, heart failure, rheumatoid, chronic obstructive pneumonia, tumor prognosis and so on. Clinicians often detect multiple inflammatory markers at ...

Result display time: 10 min - 15 min
Specificity: 99 %
Sensitivity: 98 %
... signs and symptoms of COVID-19 Product Description This Rapid antigen test kit is used for early triage and rapid management of people suspected of being infected with pneumonia ...

... Mycoplasma Pneumoniae Virus Detection Kit (Fluorescence PCR) is a qualitative in vitro real-time PCR test for the detection of nucleic acid from Mycoplasma Pneumoniae Virus. Including ...

Specificity: 88, 95 %
Sensitivity: 100, 96 %
... M.pneumoniae IgG, IgA and IgM EIA kits provide accurate results by using highly specific antigen: P1 protein The kits come with ready-to-use, well sufficient reagents and breakable, ...
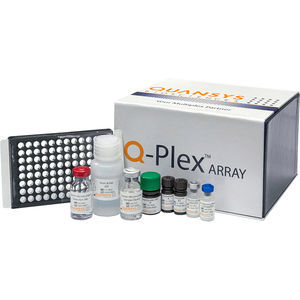
Result display time: 4 h
Sample volume: 0.005 ml
... and not all imagers are compatible with Quansys Q-Plex arrays. Kit Content Each kit contains a 96-well plate, featuring the relevant biomarker panel in each well, and all reagents ...
Quansys Biosciences
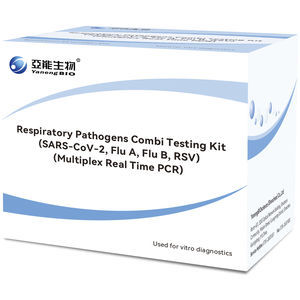
... individuals suspected of COVID19, Influenza or Respiratory Syncytial Virus pneumonia. General Specification Precision: 95% LoD: 500 copies/mL Packing Specifications 48 tests/kit

Result display time: 15 min
Sensitivity: 96, 67 %
... quick test for Legionella longbeachae. So you have clear insight for initiating the right treatment for the best possible outcome. AS EASY AS 123 Get results fast in 3 simple steps 1.INTRODUCE SAMPLE Add ...

... of 7 respiratory bacteria associated with pneumonia using real-time PCR Allplex™ PneumoBacter Assay is a multiplex real-time PCR assay to detect and identify 7 respiratory bacteria associated with pneumonia. ...

... ; SARS-CoV-2; Variant; Omicron; Flu A, Flu B; RSV; Tuberculosis; Dengue; Respiratory Pathogens Multiple Detection Kit A variety of detection kits provide reliable ...
Result display time: 10 min
This IgE (allergy) test Kit is developed to be used for the quantitative detection of total IgE in human whole blood, serum or plasma. It is for in vitro diagnostic use only, not for self-testing of untrained ...

This covid-19 PCR test kit is used for in vitro qualitative detection of the ORF1ab and N genes of novel coronavirus (2019-nCoV) from throat swabs and sputum samples in pneumonia suspected ...
DaAn Gene

... for respiratory disease, virus infection, blood bank. Such as Community-acquired of Pneumonia (CAP) including SARS-CoV-2 (COVID-19), Mycoplasma and Chlamydia Pneumonia, EB Virus and Procalcitonin, etc.

Result display time: 15 min - 15 min
Specificity: 99.3 %
Sensitivity: 94.7 %
... also be transmitted through contact. This kit is intended to be used for in-vitro qualitative detection of SARS-CoV-2 antigens in human naso-/oropharyngeal swab and saliva samples. Product description Pneumonia ...

2019-Novel Coronavirus, Influenza Virus, Respiratory Syncytial Virus Nucleic Acid Test Kit-Predicator of Respiratory Infectious Disease This kit is intended for the qualitative detection ...
Jiangsu Mole Bioscience
... of acute pneumonia in children. The development of molecular biology also draws more attention to the fluorescence quantitative PCR technology for the detection of MP-DNA. This diagnostic kit is an ...
Sansure Biotech
... SARS-CoV-2) RT-qPCR Detection Kit [N and RNAse P genes-based Assay] allows efficient cDNA synthesis and Real-Time PCR in a single tube. The kit includes a RT-qPCR MasterMix supplied in ...
Result display time: 45, 20 min
Sample volume: 0.005 ml
... concentration in serum. C- Reactive protein (CRP) was identified by Tilet and Francis (1930) in the plasma of patients with pneumonia, and was named for its ability to bind and precipitate the C-polysaccharide of pneumococcus.1,2 ...

Result display time: 45 min
... SMARTCHEK® novel coronavirus (SARS-CoV-2) detection kit which is based on biochip sample format and provides relatively short turn-around-time with simple workflow while it offers key benefits of real-time ...
Genesystem Co., Ltd.

... MERS-CoV A real time molecular test for the screening and the differentication of Influenza A and B strains, COVID-19 and MERS-CoV Product Code:GRA2032 Detection: Flu A Flu B SARS-CoV-2 MERS-CoV Specimen: Nasopharyngeal ...
Medical Innovation Ventures

【Product name】Parainfluenza virus type 1 nucleic acid detection kit(PCR-Fluorescent probe method) 【Intended use】 This kit is used for the qualitative detection of parainfluenza virus type 1 (PIV1) ...
hecin-scientific
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group


























Please specify:
Help us improve:
remaining