- Laboratory >
- Laboratory medicine >
- Parasite test kit
Parasite test kits
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}

Result display time: 65 min
... PCR run time of less than 65 minutes. - Assays available in four separate kits for increased flexibility as per laboratory requirements. QP10706: MG/TV fleX qPCR Assay - 96 Tests

Result display time: 140 min
The Sexually Transmitted Infection (STI) array is the broadest multiplex cartridge-based STI test on the market simultaneously detecting 7 bacterial, 2 viral and 1 protozoan infection for a comprehensive sexual health ...

... duration of the infection and prevent possible complications. The STAT-NAT® Leishmania spp. kit contains all the necessary reagents for Real-Time PCR analysis. STAT-NAT® contains all the reaction ...

The Aptima vaginitis panel from Hologic consists of molecular nucleic acid amplification tests (NAAT) to aid in the detection of vaginosis and vaginitis. Our assays detect the three most common causes of infectious vaginitis: ...

For in vitro diagnostic use. The RIDA®UNITY Parasitic Stool Panel II test, performed on the RIDA® UNITY platform, is a multiplex real-time PCR for the direct qualitative detection of Giardia lamblia, Cryptosporidium spp., ...
R-Biopharm AG

... Stool Panel I multiplex real-time PCR is intended for use as an aid in diagnosis of gastrointestinal infection caused by parasites. General information: Giardia lamblia, Cryptosporidium spp. and Entamoeba histolytica ...
R-Biopharm AG

... Stool Panel II multiplex real-time PCR is intended for use as an aid in diagnosis of gastrointestinal infection caused by parasites. General information: Giardia lamblia, Cryptosporidium spp. and Entamoeba histolytica ...
R-Biopharm AG

Result display time: 10 min
Sample volume: 0.01 ml
... the incubation period ranges from several weeks, several months to more than 1 year. Most dogs are in a state of recessive parasites after infection, and generally have no obvious symptoms. A small number of dogs have ...

... these instructions. -All reagents must be at room temperature before running the assay. -Do not remove test cassette from its pouch until immediately before use. -Do not reuse the test ...
Result display time: 5 min - 10 min
... to these instructions. -All reagents must be placed at room temperature before running the assay. -Do not remove test cassette from its pouch until immediately before use. -Do not reuse the test ...

Result display time: 10 min - 15 min
Sample volume: 0.01 ml
... Accuracy - about 98% Certification - ISO9001/ ISO13485 Shelf Time - 24 months Package - 25 tests/box The ToRCH IgM Antibodies Combo Test is a rapid chromatographic immunoassay for the ...

Result display time: 5 min - 10 min
... operation, cloud platform quality control. 6. The detection sensitivity is high, comparable to that of enzyme-labeled kits. • Test devices • Droppers • Single Buffer • IFU
Specificity: 98.2 %
Sensitivity: 96.4 %
... vitro diagnostic medical device for diagnosing sexually transmitted infections The careGENETM STD-12 detection kit is a real-time PCR multiplexed test intended for the simultaneous ...

EHP (Enterocytozoon hepatopenaei) is an intracellular parasite that mainly infects the hepatopancreas tissue of shrimp, therefore interfering with the shrimp from absorbing nutrients and resulting in falling production of shrimps.

Sample volume: 0.03, 0.01 ml
TORCH infections are a group of congenital infection and perinatal infection caused by pathogens, toxoplasma gondii (TOX), other pathogens,rubella virus (RV), cytomegalovirus, cytomegalovirus (CMV), herpes simplex virus(HSV) etc., which ...

Result display time: 105 min
Toxoplasma IgM is an ELISA kit for qualitative detection of IgM antibodies to Toxoplasma Gondii, in human serum, as an aid for diagnosis of active Toxoplasma infection. Clinical Significance: Toxoplasma ...

Result display time: 2 min
Specificity: 98.1 %
Sensitivity: 100 %
Toxoplasma gondii is a highly prevalent intracellular parasite responsible for toxoplasmosis in the general population, and responsible for highly severe infections in immunocompromised patients. Severe toxoplasmosis ...
ELITech Group

Result display time: 5 min
Specificity: 100 %
Sensitivity: 95, 50 %
Procleix assays expand blood screening with a comprehensive NAT assay portfolio. The NAT solutions portfolio provides screening of: HIV-1, HIV-2, HCV, HBV, WNV, Parvovirus B19, HAV, HEV, Dengue Virus, Zika Virus, Babesia, and Plasmodium. When ...
Grifols

... identification of 25 (gastrointestinal) pathogens Efficient syndromic test The syndromic screening test for efficient and cost-effectiveness patient care Proper patient care Accurate test ...
Seegene

... best possible target sequences and at the same time ensuring the kits have the broadest possible detection profile to capture all clinically relevant strains and subtypes. 150 tests per kit: ...
Cole-Parmer

... dehydrogenase (pLDH.) in human whole blood. DETECTION RANGE: APPLICABLE INSTRUMENT FiCA™ tests only can be performed with FiCA™ analyzers. STORAGE Test Kits should be ...
Medlere Limited

... antigen, IgG/IgM antibody detection reagent (colloidal gold method) Dengue virus NS1 antigen detection reagent (fluorescence immunochromatography method) Product use: Dengue virus antigen and antibody ...
Shenzhen Zijian Biotechnology Co., Ltd.
Result display time: 15 min
Specificity: 99.3, 95.8 %
Sensitivity: 89.2, 100 %
Immunochromatography test for the detection of Giardia lamblia antigens in human stool samples. Advantage Sensitive and specific Easy to use and easy to read Minimal sample manipulation

InPouch® TV is a self-contained broth media device for the recovery and detection of Trichnomonas vaginalis from female vaginal samples or male urethra/urine samples. The selective, nutritive media provides presumptive-positive results ...
Biomed Diagnostics, Inc.

sexually transmitted disease test kitGeneFinder™
Result display time: 90 min
... carryover contamination Reliable result by internal/positive/negative control Easy to use master mix type Including complete assay kits CE-IVD / Ministry Food and Drug Safety (Korea) approved
OSANG Healthcare
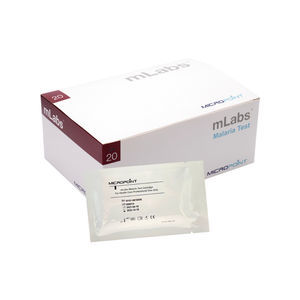
Result display time: 3 min
Sample volume: 0.015 ml
Specificity: 94.2, 99, 99.8 %
Microfluidic Immunofluorensence on mLabs® ImmunoMeter or Smart Analyzers Features Micropoint mLabs® malaria test is a microfluidic-based Immunofluorescence assay for rapid qualitative detection of Malaria P.f/Pan ...

Result display time: 60 min
Specificity: 99.2 %
Sensitivity: 98.5 %
... conventional methods by providing, faster, more accurate, and comprehensive results. 22 Targets. 1 Test. The FDA-cleared BioFire GI Panel tests for 22 of the most common pathogens associated with ...

... Immunosorbent Assay) is a test format particularly suited for the qualitative and quantitative determination of specific IgA, IgG, or IgM antibodies directed against a range of different pathogens. The following AESKULISA® ...

Regulatory Status (USA):510(k) Exempt Discipline:Infectious Disease
Gold Standard Diagnostics

Bosphore Malaria Detection Kit v1 detects, is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore Malaria Detection Kit ...
Anatolia Tani ve Biyoteknoloji Urunleri Sanayi ve Ticaret A.S.
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group
























Please specify:
Help us improve:
remaining