- Secondary care
- Cardiology
- PTA catheter
- Bard Medical
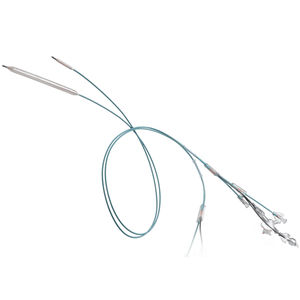
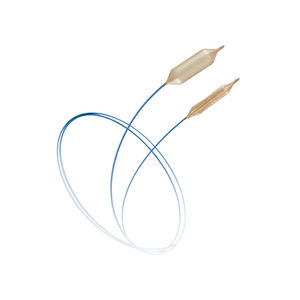
PTA catheter LUTONIX® 035blood vesselballoon
Add to favorites
Compare this product
Characteristics
- Application
- PTA
- Area of the body
- blood vessel
- Options
- balloon
Description
Lutonix™ 035 DCB – Dysfunctional AV Fistula Indication
First drug coated balloon approved for use in dysfunctional/stenosed dialysis fistulae
Features
First drug coated balloon approved for use in dysfunctional/stenosed dialysis fistulae
Shown to enable longer AV fistula function due to increased time to first reintervention compared to standard angioplasty
71.4% Primary Patency in the Lutonix™ AV Clinical Study at 6 months
31.3% fewer reinterventions than PTA at 6 months in the LUTONIX® AV Clinical Study
Demonstrated a safety profile that is as safe as PTA
Patients in the Lutonix AV IDE trial experienced 322 reintervention-free days after treatment with Lutonix, compared to 114 with PTA
The Lutonix AV Clinical Trial was the first to evaluate the use of a drug
coated balloon in dysfunctional AV fistulae. At 24 months, patients who
were treated with a Lutonix™ 035 Drug Coated Balloon PTA Catheter
went an average of 322 days before needing a reintervention compared
to 207 days when treated with PTA alone*.
· Broadest Size Offering for AV DCBs†
· Lowest Profile AV DCB with All Sizes 7F or Lower
· More Intervention-Free Days than Standard PTA
Lutonix™
035 Drug Coated Balloon PTA Catheter
Indications for Use: The Lutonix™
Catheter is indicated for percutaneous transluminal
angioplasty (PTA), after pre-dilatation, for treatment of stenotic lesions of dysfunctional
native arteriovenous dialysis fistulae that are 4 mm to 12 mm in diameter and up to 80 mm
in length.
Catalogs
No catalogs are available for this product.
See all of Bard Medical‘s catalogsRelated Searches
- Catheter
- Balloon catheter
- Stent
- Dilatation catheter
- Peripheral catheter
- Cardiac catheter
- Metal stent
- Catheter guidewire
- Diagnostic catheter
- Access catheter
- Venous catheter
- Central venous catheter
- Hydrophilic guidewire
- Blood vessel catheter
- PTA catheter
- Nitinol stent
- Triple-lumen catheter
- Self-expanding stent
- Vascular introducer
- Balloon catheter pump
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.