- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
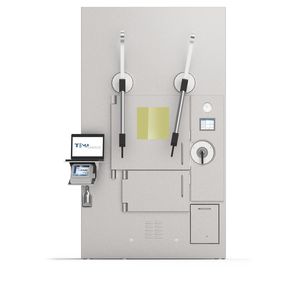
Research and development shielded cell RES 1for radiopharmacysynthesisshielded
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Applications
- synthesis, for radiopharmacy, for research and development
- Other characteristics
- shielded
- Air flow type
- vented
- Structure material
- polycarbonate
Description
RES 1 is a GMP grade B isolator for radiopharmaceutical research, with turbulent flow involving the whole working area.
Radiopharmaceuticals synthesis and manipulations take place in a controlled and safe environment as RES 1 has been specifically designed to comply to the latest GMP guidelines. Its inflatable seals are granting an ISO 10648:2 air tightness.
A smart ventilation system keeps both the pressure inside the hot cell and the air exchange constant, independently from building HVAC and filter clogging, and even in cases of:
non efficient internal leak tightness
breaking of the gloves
breaking of the teletong booting.
KEY FEATURES
main shielded front door with n.2 shielded hatches (glove ports) + polycarbonate front door with gloves
extractable tray and removable support shelf for ancillary equipment storage
n.1 air-tight, shielded and vented pre-chamber + n.1 teletong
vial extraction system (double door tunnel) + drawer for lead pot retrieval with automatic lid positioning
integrated shielded area for dose calibrator
protective coating against corrosive agents in the internal area (option)
suspended sliding lead glass window in the main working area (option)
Catalogs
No catalogs are available for this product.
See all of Tema Sinergie‘s catalogsRelated Searches
- Biological safety cabinet
- Laboratory biological safety cabinet
- Class II biological safety cabinet
- TEMA SINERGIE aseptic isolator
- TEMA SINERGIE floor-standing isolator
- Floor-standing biological safety cabinet
- Class III isolation chamber
- Pass-through
- TEMA SINERGIE containment isolator
- Cleanroom pass box
- TEMA SINERGIE pharmaceutical isolator
- TEMA SINERGIE isolator with HEPA filter
- Hospital monitoring system
- TEMA SINERGIE negative pressure isolator
- Laminar flow biosafety cabinet
- TEMA SINERGIE stainless steel isolator
- TEMA SINERGIE transfer isolator
- TEMA SINERGIE dispensing isolator
- Temperature monitoring system
- Laminar flow isolator
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.