- Laboratory
- Laboratory medicine
- Pneumonia test kit
- Zhejiang Orient Gene

- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
Pneumonia test kit 101A0207EYvirusMycoplasmanasopharyngeal
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Applications
- pneumonia
- Micro-organism
- virus, Mycoplasma
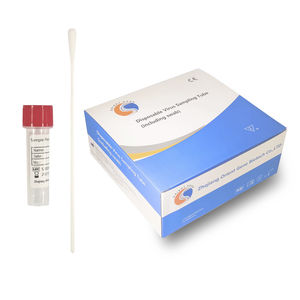
- Sample type
- nasopharyngeal
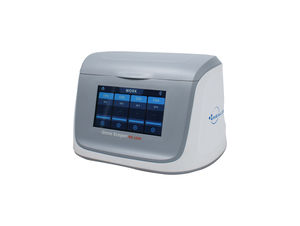
- Analysis mode
- real-time, fluorescence, for PCR, for real-time PCR
- Format
- lyophilized
Description
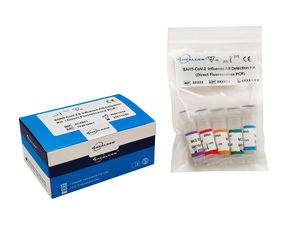
The Mycoplasma Pneumoniae Virus Detection Kit (Fluorescence PCR) is a qualitative in vitro real-time PCR test for the detection of nucleic acid from Mycoplasma Pneumoniae Virus.
Including Generic reagent and Lyophilized reagent
Specifications:
Analytical Sensitivity (LoD):1×103copies/mL
Sample Type: Oropharyngeal swab or nasopharyngeal swab
Protocol Duration: 100 minutes
Storage: 101A0207EY:-25℃ to -15℃
101A0207ED (Lyophilization): 0°C to 35°C
Shelf Life: 101A0207EY12 months from the date of manufacture
101A0207ED (Lyophilization): 18 months from the date of manufacture
Catalogs
No catalogs are available for this product.
See all of Zhejiang Orient Gene‘s catalogsRelated Searches
- Assay kit
- Blood assay kit
- Molecular biology reagent kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Cassette rapid diagnostic test
- Molecular test kit
- Virus rapid diagnostic test
- Serum rapid diagnostic test
- Whole blood detection kit
- Respiratory infection test kit
- Plasma rapid diagnostic test
- Infectious disease rapid diagnostic test
- Optical assay kit
- Clinical assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.