- Secondary care >
- Oncology >
- Implantable titanium port >
- B Braun
B Braun implantable titanium ports
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}

... sampling or transfusion. The port has an epoxy body with a titanium chamber and is high pressure resistant up to 325 PSI (22,4 bar). Special Features - Compact port design with enlarged ...
Aesculap®

... CT" marking (except for the US market) which indicates power injectability of the port on x-ray - Compact port design with enlarged puncture area in relation to the port housing size - ...
Aesculap®

Celsite® Discreet Venous access ports with unique design for enhanced port stability and better cosmetic results Celsite® Discreet Access Port Systems are indicated for any condition ...
Aesculap®

TITAN-PORT D (dialysis) is a fully implantable venous port catheter system as an access option for performing veno-venous dialysis. There are 2 different systems available in complete ...
PakuMed Medical Products

TITAN-PORT APH (exctracorporeal apheresis) is a fully implantable port catheter system providing an access facility for performing extracorporeal apheresis. The set contains a titanium ...
PakuMed Medical Products

... punch defects. Technical failures excluded » Safety increased. The TITAN-PORT G System is a totally implantable port catheter system consisting of an injection chamber (port), ...
PakuMed Medical Products

... PowerFlow™ Implantable Apheresis IV Port The PowerFlow™ Implantable Apheresis IV Port is the first and only high-flow, power-injectable port designed ...
Bard Medical

Lightweight for patient comfortEasily identifiablePower injectableTitanium port body
Bard Access Systems

The SmartPort+ implantable port is the latest in implantable port innovation from AngioDynamics and combines two of our best-in-class technologies, Vortex and Endexo, ...
Angiodynamics

Weight (g) :14 Height x base (mm) :12 x 30 ø puncture (mm) :13 Residual Volume (ml) :0,5
Districlass médical
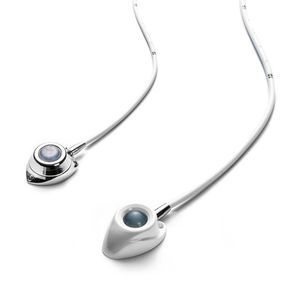
... cm peel-away with a 0,018" Nitinol guide wire and 21G needle. Produced to meet the highest quality standards, the implantable Port systems allow long-term medication ...

... to lead a normal day life between infusions. The INFU-KT port consists of a compact titanium injection port, with a self-sealing silicone septum and a silicone delivery catheter. ...
F.B. Medical

... Compatible with pressure injections for CT scan polysite size procedures Titanium and Polyoxymethylene ports Hybrid ports : Titanium & POM (polyoxymethylene) ...
Perouse Medical

Vaxcel® Implantable Ports Vaxcel® Implantable Ports is a thin, soft, plastic tube that is put into a vein in your chest or arm and has an opening (port) ...
Navilyst Medical

... Especially indicated for treatment with vasoactive or irritant drugs. Description Titanium port, with embossed silicone septum, to facilitate its location Low profile port. Greater ...
Your suggestions for improvement:
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group












Please specify:
Help us improve:
remaining