- Laboratory >
- Laboratory medicine >
- Liver disease test kit
Liver disease test kits
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
... or EDTA plasma samples. Additional subtype information is available in a majority of cases. The INNO-LiPA HCV 2.0 Amp kit, for in vitro diagnostic use, is designed for use in reverse transcription and amplification ...
Fujirebio

For research use with the automated LUMIPULSE G System for the quantitative measurement of protein induced by vitamin K absence or antagonists-II (PIVKA-II) in human serum. This product is not for use in diagnostic procedures, for ...
Fujirebio

... Low indeterminate rate High sensitivity in BBI panels Short and overnight procedures Color-coded reagents Ready-to-use reagents Four control lines to monitor 1) non-specific reactivity, ...
Fujirebio
... type 1+2 human immunodeficiency virus (HIV-1 and HIV-2) in plasma/serum specimens. The specifications are available in 96 tests/box, supporting three-time usage, with a 100% report output rate.
Shanghai Kehua Bio-engineering

Specificity: 100 %
Sensitivity: 100 %
Colloidal gold products are used before emergency operation, entry-exit inspection and quarantine bureau, health examination and before invasive examination, easy and fast to use. KHB anti-HIV colloid gold product has been included ...
Shanghai Kehua Bio-engineering

... or plasma samples. This is used for assessment of response to antiviral therapy and monitoring of therapeutic effect. This kit is not used fo blood screening.
Shanghai Kehua Bio-engineering
Specificity: 99.9 %
Sensitivity: 100 %
Deliver trusted results with dual targets across a wide linear range and all major HBV genotypes. The Aptima® HBV Quant Assay is a molecular HBV treatment management assay with dual-target design. It helps providers guide treatment ...
Result display time: 10 min - 15 min
Sample volume: 5 ml
... HCV HbsAg combo Test Device(whole blood,serum and plasma)is a rapid chromatographic immunoassay for the quantitative detection of antibodies to HIV,HCV,HbsAg in whole blood,serum and plasma. Intend to use The HIV ...
Result display time: 15 min
Sample volume: 0.1 ml
... colored, it means that the test result is invalid regardless of whether the test line is colored. The control line is the criterion for judging whether the test strip is valid. [Materials ...
Result display time: 3 min
... assay is intended for in vitro quantitative determination of C-Reactive Protein in human serum, plasma and whole blood. The test kit is used as an aid in the diagnosis of infection and inflammation. For ...
PIIINP Detection Kit (CLIA) CIV Detection Kit (CLIA) LN Detection Kit (CLIA) HA Detection Kit ...
Sample volume: 0.1, 0.01, 0.05, 1, 0.075 ml
Infectious diseases are diseases that can be transmitted among people and become pandemic, which infect another person or animal species through various routes. Infectious diseases are ...
Autobio Diagnostics
Result display time: 5 min
Specificity: 95 % - 100 %
Sensitivity: 95, 50 %
... collected in K2EDTA, K3EDTA, or in a gel separation tube Pool sizes IDT-NAT, Pools of 4, 8, 16, or 96 Assay kit size 1000 or 5000 tests Detected virus HIV-1 group M ...
Grifols
Result display time: 2 min
Specificity: 97.6 %
Sensitivity: 100 %
Hepatitis B is a liver infection caused by the hepatitis B virus (HBV). Treatment can slow the progression of cirrhosis, reduce incidence of liver cancer and improve long term survival. Most people who ...
ELITech Group
Result display time: 105 min
Sample volume: 0.01 ml
... cholangitis (PSC) is a slowly developing disease of the gall bladder that predominantly affects young adults up to middle age and in a significant proportion of cases leads to inflammation of the liver ...
MEDIPAN GmbH

... Name: Hepatitis B Virus Detection Kit (Fluorescence PCR) Sample Type: Plasma (EDTA anticoagulant) or serum Assay Target: Hepatitis B virus DNA (genotypes A-H) Analytical Sensitivity(LoD): 20 IU/mL Specification: ...
Zhejiang Orient Gene

... best possible target sequences and at the same time ensuring the kits have the broadest possible detection profile to capture all clinically relevant strains and subtypes. 150 tests per kit: ...

... medical and health institutions for the screening of infectious diseases with Hepatitis B virus (HBV). For in vitro diagnostic use only. For professional use only. TEST PRINCIPLE This kit ...
Medlere Limited

Up to 17 parameters in one test strip Comprehensive test panels for the detection of ANA, ALD, Vasculitis, Myositis, and Diabetes Unique control band on test strips can generate ...

Result display time: 60 min
... Detection Colorimetric The Anti-HAV IgG ELISA is an enzyme immunoassay for the qualitative detection of the Hepatitis A virus antibody (Anti-HAV) in human serum or plasma. The Anti-HAV IgG ELISA ...
ALPCO

... High-efficient quality control system HBV DNA test kit (MB) uses UNG enzyme + dUTP system to remove carry-over contamination to avoid a false positive result. HBV DNA test kit ...
Sansure Biotech

In liver diseases, an accurate differential diagnosis between viral and autoimmune hepatitis is crucial for the choice of an appropriate therapy. Autoimmune liver diseases ...
AESKU.GROUP
... Quantitative RT-PCR Kit is an in vitro diagnostic kit designed for the quantification of hepatitis C virus (HCV) RNA in human samples such as serum and EDTA-plasma through real-time PCR. Product Description Hepatitis ...

Bosphore HBV Detection Kit v1 detects hepatitis B virus DNA in human serum or plasma, encompassing all the major HBV genotypes (A-H). A region within the S gene is amplified and fluorescence detection ...

Kit for the detection of Hepatitis C virus (HCV) genotypes 1 to 7 and subtypes a and b of genotype 1 by reverse transcription, PCR and Reverse Line Blot of the 5’UTR and CORE region. The kit can accurately ...

... Chronic hepatitis may progress to liver scarring (cirrhosis), liver failure, and liver cancer. Viral Hepatitis presents global health challenges, with death rates beginning to outnumber ...

Result display time: 15 min - 20 min
Product Name HWTS-OT086-HIV Ag/Ab Combined Detection Kit (Colloidal Gold) HWTS-OT087-HIV Ag/Ab Combined Detection Kit (Colloidal Gold) Epidemiology Human immunodeficiency ...
Jiangsu Macro micro-test Medical Technology

... spectrum of clinical manifestations ranging from mild, inapparent disease to fulminant hepatitis, severe chronic liver diseases, which in some cases can lead to cirrhosis and carcinoma ...
ADALTIS
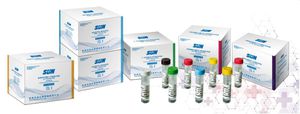
Quantitative determination of Hepatitis B virus surface Antigen (HBSAG) in human serum in vitro
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group
























Please specify:
Help us improve:
remaining