- Laboratory >
- Laboratory medicine >
- Virus test kit
Virus test kits

Result display time: 90 min
... with HPV infection being its primary cause. Persistent HPV infections can progress to cancer over 10–15 years. Early diagnosis through HPV testing is crucial. The PaxView® HPV ...
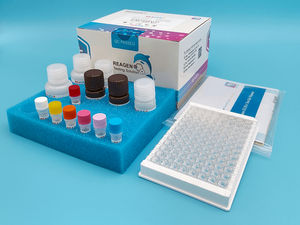
... Titration ELISA Test Kit is a competitive enzyme immunoassay for the quantitative determination of AAV serotype 8 particles in cell culture supernatants and purified virus preparations. Product ...
Reagen

Mmiso® FMDV pan Detection Kit is designed to amplify seven serotypes: O, A, Asia1, C, SAT1, SAT2 and SAT3 of the foot and mouth disease virus under isothermal conditions combined with ...

ELISA testing is an effective and accurate method for detecting the COVID-19 infection, ideally suited for laboratories.
Sample volume: 0.005 ml
The SC2 Real-Time PCR Detection Kit is designed for specific and qualitative detection of SARS-CoV-2 RNA in samples such as oropharyngeal swabs, nasopharyngeal swabs or sputum suspected of SARS-CoV-2. Principle The ...
Biomiga, Inc.
Result display time: 10 min
Specificity: 99.7 %
Sensitivity: 99.3 %
Human immunodeficiency virus, or HIV, is a pathogen that attacks and suppresses the immune system by specifically affecting white blood cells. read more WHO IS THIS TEST FOR? HIV 1/2 SELF-TEST ...

Result display time: 15 min
Sample volume: 0.1 ml
... binding to the ACE2 receptor of human cells. High levels of nAbs indicate immunity against COVID-19. Standard IgG/IgM antibody tests – which are universally available – do not detect for nAbs and can only provide an indirect ...
... pap-smear test done on time. It will be a life saver for all women who cannot and donnot want have their pap-smear test done by classical methods, because EARLY DIAGNOSIS SAVE LIVES. Why Easy Smear? It ...
Specificity: 99.9 %
Sensitivity: 80.2 %
... (SARS-CoV-2) pandemic, Bio-Helix has specifically developed the LifeDireX COVID-19 RT-qPCR Detection Kit Plus for human respiratory tract specimens. The kit is characterized by: (1) ...

... Sealing Tape:, 3 Sheets Storage - 2 - 8 °C Shelf Life - 2 months from date of receipt Production Procedures - A complete kit for determining SARS-CoV-2 IgG levels in samples.

... vitro diagnostic kit used for the detection of Monkeypox Virus Nucleic Acids. The kit can be used to assess samples from skin swabs, papule or vesicle. The kit specifically ...
Result display time: 10 min
The CareStart™ COVID-19 Antigen Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigens from SARS-CoV-2. – Fast and easy to self-test at anywhere – ...
Specificity: 100 %
Sensitivity: 100 %
... and other laboratory test outcomes. The product is intended for use by healthcare professionals specifically trained in nucleic acid amplification techniques and in vitro diagnostic procedures. The COVID-19 Real-Time ...

One Step immunochromatographic assay for the qualitative detection of SARS-CoV-2 Antigen using nasopharyngeal swabs taken from patient showing symptoms of COVID-19 infection.
Quantitative determination of Hepatitis B virus surface Antigen (HBSAG) in human serum in vitro

Result display time: 15 min
• Evaluate neutralizing antibody after COVID 19 vaccine is injected. After injection, neutralizing antibodies will be produced in the human body, and the effffect after the vaccine injection and the level of neutralizing antibodies in ...
Neutralizing Antibody test is used to assess the immunity status of the patient against COVID-19. Unlike a general antibody screen, it spesifically detects the neutralising antibodies.

The ImmTekTM COVID-19 product line aims to ease the burden of diagnosis on health facilities. We hope each product category can play a critical and complementary role in response to COVID-19 pandemic from early-stage detection to epidemiological ...

... reaction with other coronavirus • Compatible with mainstream qPCR devices COVID-2019 Virus Nucleic Acid Detection Kit(Dual-RTPCR) 【Product Introduction】 This kit is used for nucleic ...

... Ureaplasma(U. urealyticum & U. parvum)qPCR Detection kit Introduction MG, MH,UU and UP are kinds of infectious diseases. The inflection of them can cause urethritisand genital tract inflammation. This ...

Result display time: 80 min
ELISA kit for the qualitative detection of IgG antibodies to SARS-CoV-2 virus Characteristic: Number of tests — 96 Expiration date — 12 monthes Storage temperature ...
Equi

The Enterovirus RNA Real-Time PCR kit coded ENTERORNA.CE is intended for the qualitative detection of Enterovirus RNA in human plasma and CSF samples (Cerebral Spinal Fluid) with a simultaneous control of the amplification ...
... ) as positive control. Target Gene - Test/kit - Storage E gene - 100 tests - -20°C (±3°C) Workflow Oropharyngeal swab Sample collection ...

Detection Kit for Monkeypox Virus ( Real-time PCR ) is designed for the qualitative detection of Monkeypox Virus (MPV) DNA extracted from skin, fluid, or crusts collected ...
Xiamen Biotime Biotechnology Co., Ltd.
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group

























Please specify:
Help us improve:
remaining