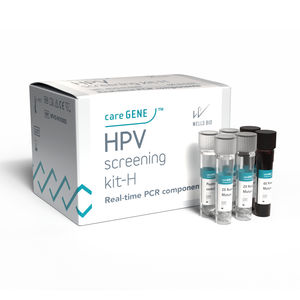
Sexually transmitted disease assay kit careGENE™ HPV detection kit-Mfor cervical cancerHPV 33HPV 16
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Applications
- for sexually transmitted diseases, for cervical cancer
- Micro-organism
- HPV 33, HPV 16, HPV, HPV 59, HPV 68, HPV 61, HPV 18, HPV 40, HPV 31, HPV 35, HPV 58, HPV 44, HPV 39, HPV 66, HPV 53, HPV 70, HPV 45, HPV 51, HPV 52, HPV 56, HPV 43, HPV 6, HPV 54, HPV 81, HPV 11
- Sample type
- for cervical cells
- Analysis mode
- for PCR
- Specificity
98.1 %
- Sensitivity
90 %
Description
INTENDED USE
In vitro diagnostic medical device for screening of human papillomavirus (HPV).
The careGENETM HPV detection kit-M is designed to detect 25 types of human papillomavirus types (HPV). In conjunction with qPCR, it can genotype 4 high-risk HPVs (16, 18, 31, and 59) and 3 low-risk HPVs (6, 53, and 61). It can collectively detect 10 other high-risk HPVs (HPV 33, 35, 39, 45, 51, 52, 56, 58, 66, and 68), and 8 other low-risk HPVs (HPV 11, 40, 43, 44, 54, 70, 73, and 81) from crude cervical scraping (Liquid based Cytology).
KEY FEATURES AND BENEFITS
√ Limit of Detection ≥ 10 copies/rxn
√ Verification of sample extraction and amplification efficiency using endogenous internal control
√ High analytical sensitivity
√ Uracil-DNA Glycosylase (UDG) system applied
√ Compatibility – Flexible open system
Catalogs
No catalogs are available for this product.
See all of WELLS BIO‘s catalogsExhibitions
Meet this supplier at the following exhibition(s):

27th European Society For Clinical Virology
17-20 Sep 2025 Thessaloniki (Greece) Stand E12
More information
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Cassette rapid diagnostic test
- Molecular test kit
- Virus rapid diagnostic test
- Serum rapid diagnostic test
- Whole blood detection kit
- Respiratory infection test kit
- Plasma rapid diagnostic test
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- COVID-19 detection kit
- Rapid respiratory infection test
- Real-time PCR test kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.