- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
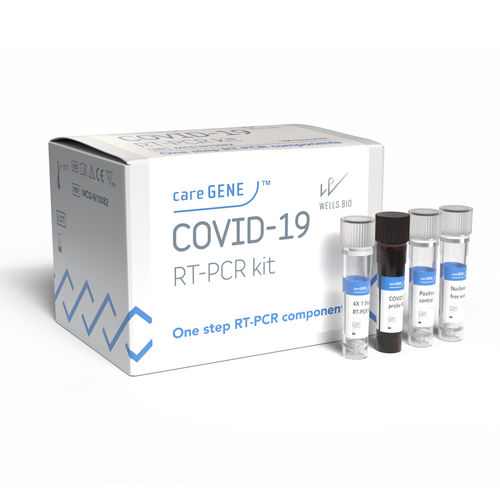
RT-PCR detection kit careGENE™ COVID-19 kitfor infectious diseasesCOVID-19SARS-COV-2

Add to favorites
Compare this product
Characteristics
- Applications
- for infectious diseases, COVID-19
- Micro-organism
- SARS-COV-2
- Sample type
- nasopharyngeal, sputum
- Analysis mode
- real-time, for RT-PCR, for qPCR, RT-qPCR
- Result display time
83 min
- Sample volume
0.01 ml
(0.00034 US fl oz)- Specificity
100 %
- Sensitivity
99.3 %
Description
INTENDED USE
in vitro diagnostic medical device for detection of COVID-19
careGENE™ COVID-19 RT PCR kit is an in vitro diagnostic medical device for qualitative detection of coronavirus disease (COVID-19) from RNA extracted from human nasoparyngeal swab, oropharyngeal swab, and sputum using real-time RT-PCR
KEY FEATURES AND BENEFITS
√ Limit of Detection: 10 copies/µL
√ Compatibility – Flexible open system
VIDEO
Catalogs
No catalogs are available for this product.
See all of WELLS BIO‘s catalogsRelated Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Cassette rapid diagnostic test
- Molecular test kit
- Virus rapid diagnostic test
- Whole blood detection kit
- Serum rapid diagnostic test
- Respiratory infection test kit
- Plasma rapid diagnostic test
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- COVID-19 detection kit
- Rapid respiratory infection test
- Real-time PCR test kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.