- Laboratory >
- Laboratory medicine >
- QPCR test kit
QPCR test kits
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}

Result display time: 65 min
... run time of less than 65 minutes. - Assays available in four separate kits for increased flexibility as per laboratory requirements. - QP10705: HSV1/HSV2/TP fleX qPCR Assay - 96 Tests
Result display time: 40 min
Real-time RT-PCR test for the fast qualitative detection of SARS-CoV-2 (N, ORF1a and RdRp) nucleic acids in various sample types. Details Features: Hot Start PCR: high specificity Detection Target: N, ORF1a ...
Fujirebio

Result display time: 75 min - 90 min
Sample volume: 0.025 ml
... swabs without pre-treatment. Fast. Simple. Accurate. Special Features: Method: Isothermal amplification with real time fluorescence detection Simplicity: Sample does not require pre-heating, RNA extraction ...
Fujirebio
A real-time fluorescent RT-isothermal assay based on Atila’s proprietary isothermal amplification technology intended for the qualitative detection of nucleic acid from the SARS-CoV-2 in nasopharyngeal swabs, oropharyngeal swabs and saliva ...
Fujirebio

... technologies Product Details The therascreen EGFR RGQ PCR Kit is an FDA-approved, qualitative real-time PCR assay for the detection of specific mutations in the EGFR oncogene. The kit provides reagents ...
QIAGEN

Result display time: 50 min - 90 min
... 18 account for 70% of cervical cancer worldwide. Tianlong’s Human Papillomavirus (HPV) Nucleic Acid Detection Kit is a qualitative in vitro test for detection of 18 high-risk HPV ...
Xian Tianlong Science and Technology

SARS-CoV-2 Nucleic Acid Detection Kit (Direct Real-time RT-PCR Method) Intended Use The Tianlong SARS-CoV-2 Nucleic Acid Detection Kit is intended for the qualitative ...
Xian Tianlong Science and Technology

... Virus Nucleic Acid Detection Kit is intended for use by qualified clinical laboratory professionals trained in the techniques of Real-time PCR and in vitro diagnostic procedures. The TianLong Monkeypox ...
Xian Tianlong Science and Technology
... type 1+2 human immunodeficiency virus (HIV-1 and HIV-2) in plasma/serum specimens. The specifications are available in 96 tests/box, supporting three-time usage, with a 100% report output rate.
All-in-one qPCR kit for simultaneous detection of B. anthracis, Y. pestis and F. tularensis. Biotoxis is a qPCR all-in-one detection kit that detects ...

... duration of the infection and prevent possible complications. The STAT-NAT® Leishmania spp. kit contains all the necessary reagents for Real-Time PCR analysis. STAT-NAT® contains all the reaction ...

Result display time: 30 min
Sample volume: 0.02 ml - 0.2 ml
... Ordering Options (online, email, or phone) Volume Discounts Available on Large Quantity Orders* Blood DNA Isolation Kits These kits allow for the isolation of DNA from the blood of various species, ...
Norgen Biotek
Specificity: 99.1 %
Sensitivity: 98.3 %
... device for screening of human papillomavirus (HPV). The careGENETM HPV screening kit is test reagents for screening of human papillomavirus (HPV). In conjunction with qPCR, ...
WELLS BIO
INTENDED USE In vitro diagnostic medical device for diagnosing Pneumonia careGENE™ Pneumonia detection kit is a product that can qualitatively detect pneumonia-causing bacteria by amplifying pneumonia-causing ...
WELLS BIO
Result display time: 83 min
Sample volume: 0.01 ml
Specificity: 100 %
INTENDED USE in vitro diagnostic medical device for detection of COVID-19 careGENE™ COVID-19 RT PCR kit is an in vitro diagnostic medical device for qualitative detection of coronavirus disease (COVID-19) from RNA ...
WELLS BIO

Result display time: 45 min
... SMARTCHEK® novel coronavirus (SARS-CoV-2) detection kit which is based on biochip sample format and provides relatively short turn-around-time with simple workflow while it offers key benefits of real-time ...
Genesystem Co., Ltd.

Result display time: 40 min
... detection, enabling the simultaneous detection of all significant respiratory viral targets. SMARTCHEK® SARS-CoV-2/Flu/RSV Detection Kit is a reverse transcription real-time polymerase chain reaction ...
Genesystem Co., Ltd.

Result display time: 40 min
Simple workflow for minimized user handling with enhanced testing capacity (8 tests/run) Excellent testing performance assured by high sensitivity and specificity Assay is designed to detect IS6110 gene and MPB64 gene ...
Genesystem Co., Ltd.
For in vitro diagnostic use. The RIDA®UNITY Viral Stool Panel II test, performed on the RIDA®UNITY platform, is a multiplex real-time RT-PCR for the direct qualitative detection and differentiation of rotavirus RNA, astrovirus ...
R-Biopharm AG

For in vitro diagnostic use. The RIDA®UNITY Parasitic Stool Panel II test, performed on the RIDA® UNITY platform, is a multiplex real-time PCR for the direct qualitative detection of Giardia lamblia, Cryptosporidium spp., ...
R-Biopharm AG
For in vitro diagnostic use. The RIDA®UNITY Norovirus I & II test, performed on the RIDA®UNITY platform, is a multiplex real-time RT-PCR for the direct qualitative detection and differentiation of norovirus RNA of genogroups ...
R-Biopharm AG

Result display time: 3 h
... DNA FFPE One-Step Kit is designed for purification of total DNA from FFPE tissues by using MagCore® instruments. It features the method, One-Step Heating, to melt paraffin and lyse tissue samples at the same time without ...
RBC Bioscience
Result display time: 4 h
Specificity: 100 %
Sensitivity: 98.6 % - 100 %
NeoPlex™ STI-14 Detection Kit simultaneously detects 14 STI-causing pathogens in a single-tube multiplex real-time PCR based on proprietary C-Tag™ technology. Feature of Product Single-Tube Multiplex ...

Result display time: 2 min
Specificity: 100 %
Sensitivity: 98.5, 100 %
... detection by genetic testing is important in patients with hypercoagulability to provide adapted therapy. Coagulation ELITe MGB® Kit is a real-time PCR assay designed for the detection and the allelic discrimination of ...
ELITech Group
... quantifying baculovirus in cell culture supernatants. The test involves applying only 20 µl of precious viral supernatant to a GoStix cassette and waiting 10 minutes for the appearance of test and control ...
... Cross-reactions FAM VIC 36 CE. DOC.MSDS lOOOcopies/mL -20°C ± 5X Under frozen condition 12 Months 25 tests/k't, 50 tests/kit Nucleic acid amplification reaction ...
BEIJING APPLIED BIOLOGICAL TECHNOLOGIES

Product Name: SARS-CoV-2 Detection Kit (Direct Fluorescence PCR) Sample Type: Oropharyngeal swab or Nasopharyngeal swab Target Gene: ORF1ab Gene, N Gene Analytical Sensitivity(LoD): 200 copies/mL Specification: ...
Zhejiang Orient Gene

Specificity: 99.3, 96.9, 98.9 %
... technologies are utilized. This kit is the only CFDA-approved PCR assay for rapidly separating MTB cases from those caused by other mycobacterium species. Features of Mycobacteria Nucleic Acid Detection Get through ...

... possible target sequences and at the same time ensuring the kits have the broadest possible detection profile to capture all clinically relevant strains and subtypes. 150 tests per kit: ...
Cole-Parmer
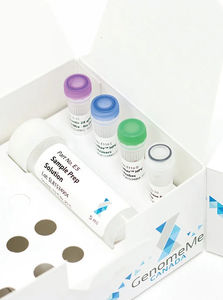
... HPV One qPCR Kit is designed for quick initial screening of individuals for the presence of all 14 High Risk human papilloma virus (HPV) subtypes allowing physicians to identify those at risk for cervical ...
GenomeMe Labs Inc
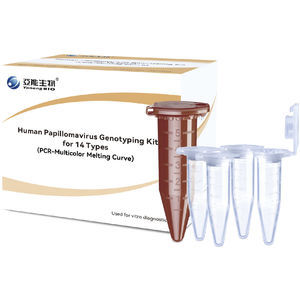
... LoD: 1×106 copies/mL Packing Specifications 24 tests/kit (non-single test/tube) - 48 tests/kit (non-single test/tube) - 96 tests/lit ...
Yaneng Bioscience (Shenzhen) Co., Ltd.

... single channel on real-time PCR instrument. Allplex™ Respiratory Panel Assays allows faster, more reliable and comprehensive test results than any other products by combination with Seegene’s automation platforms. KEY ...
Seegene

Result display time: 67 min
This kit is used for the qualitative detection of MPV in serum or lesion exudate samples. About Monkeypox Monkeypox is a viral zoonotic disease caused by monkeypox virus. It occurs primarily in tropical rainforest ...
Getein Biotech Inc.

... Diagenode’s MagMeDIP qPCR Kit has been largely inspired by the reproducibility standards reached using the SX-8G IP-Star® Automated System. If you are interested in automating this protocol, please check ...

... pneumoniae from isolated DNA sample. Principle of the method This diagnostic kit is based on RealTime PCR method using Molecular Beacons hybridization probes. In this kit for the detection of Mycoplasma ...
Elisabeth Pharmacon spol
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group

























Please specify:
Help us improve:
remaining