- Laboratory >
- Laboratory medicine >
- Respiratory disease test kit
Respiratory disease test kits

Result display time: 15 min
Sample volume: 0.1 ml
... immunity against COVID-19. Standard IgG/IgM antibody tests – which are universally available – do not detect for nAbs and can only provide an indirect indication for immunity. A SARS-CoV-2 nAb test ...
OptiBio Co., Ltd.
Result display time: 15 min
... in minutes in order to enable accurate clinical decisions. Improving access : Rapid diagnostic tests facilitate access to healthcare globally. Content: 20 tests (including positive control) Also ...
BIOSYNEX

Result display time: 15 min - 30 min
Intended Use: This assay kit is used for in vitro qualitative detection of the N gene and ORF1ab gene of novel coronavirus SARS-CoV-2 in nasal swab samples from suspected pneumonia cases of SARS-CoV-2 ...
Pluslife Biotech Co., Ltd.

Result display time: 15 min - 20 min
Sample volume: 0.01, 0.02 ml
... temperature No cold chain transportation needed. [detection principle] The kit is based on the principle of colloidal gold immunochromatography. [main components] Test card: sample pad, ...
Beijing Zhongjian Antai Diagnosis Technology Co., Ltd.

Result display time: 15 min
Specificity: 99.6 %
Sensitivity: 97.1 %
An in vitro multiplexreal-time RT-PCR test for quantitative detection of nucleic acid fromSARS-CoV-2 in oropharyngeal swab, nasopharyngeal swab or deep cough sputumspecimens. --- Single-well, triple target assay covering ...

Vitassay qPCR kits are ready to use real-time PCR assays designed to amplify and detect pathogen. Vitassay qPCR Kits contains in each well enzymes, buffer, primer and probes as well as an internal ...
VITASSAY HEALTHCARE S.L.

Result display time: 10 min
Sample volume: 0.02 ml
... immune system to provide protectionagainst the 2019-nCoV. Some patients with negative results in nucleic acid test show positive in IgM test,indicating that the IgG / IgM detection is one of the effective ...
Nanjing Vazyme Medical Technology Co.,Ltd

Result display time: 15 min
Specificity: 99.2, 99.4, 99.5 %
Sensitivity: 97.6, 94.4, 97.8 %
... causes of acute respiratory ilnesses. Symptoms of influenza are not specific and may include sudden cough, fever, weakness and sore throat which may last 2-3 weeks. Diagnostic tests available for detection ...
Turklab Tibbi Malzemeler San. Tic. A.S.
... ready-to-use PCR kit is available in lyophilized format (freeze-drying process) for long-term storage. The kit can be transported and stored at room temperature and is stable for one year. Each tube of ...
Nanjing Liming Bio-products Co., Ltd.

Result display time: 100 min - 119 min
Specificity: 99 %
Sensitivity: 100 %
... confidence High volume manufacturing capabilities – Meet global test demands >10,000 tests/hour The kit is intended to use viral RNA samples that have been pre-extracted from specimens ...

Result display time: 15 min - 25 min
... Samples: nasopharyngeal swab, oropharyngeal swab and anterior nasal swab Package specification: 5 Tests / kit, 25 Tests / kit, 50 Tests / kit
Hangzhou Bigfish Bio-tech Co., Ltd.

Result display time: 1 h
Specificity: 100 %
Sensitivity: 97.8 %
The VERI-Q SARS-CoV-2 Neutralization Antibody Detection Kit is a qualitative immunoassay for the detection of neutralization antibodies against SARS-CoV-2.

Immediate on-site Covid 19 Antigen Testing. BIOCARD Pro COVID-19 Rapid Antigen kit is a chromatographic immunoassay for the qualitative detection of specific antigens to COVID-19 present in human nasopharyngeal swab
Trivitron Healthcare

Result display time: 15 min
SARS-CoV-2 Antigen Fast Test Kit (Immunofluorescence ) is intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in human nasal swab samples from patients suspected of ...
Sichuan Xincheng Biological Co., LTD
... Nucleic Acid Test Kit (RT-PCR) is a qualitative in vitro nucleic acid amplification assay used to detect SARS-CoV-2 using reverse transcription PCR from nasopharyngeal swabs, bronchoalveolar lavage fluids ...
Result display time: 15 min
Sample volume: 0.02 ml
Vital Test Pro COVID-19 (IgG/IgM) is an atibodies capture immunochromatographic test for a simultaneous detection and differenciation of IgM and IgG antibodies to COVID-19 in human serum, plasma or whole ...
SPA VITAL CARE Production, Distribution et Logistique
10 tests/ kit registration number:CE Test Device (10 tests) Lysis Solution (12ml) Extraction Tube (10) Sterile Nasal Swab (10) Intended use Flu A qualitative ...

... in molecular testing, e.g. infectious diseases, oncology and new-born diagnostics. FELUDA is developed indigenously by CSIR-IGIB, a leading Indian biosciences research institute. Tata MD CHECK RT-PCR CRISPR test ...
Result display time: 15 min
Specificity: 99.5, 99 %
Sensitivity: 97.8, 97.2 %
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has been recognized as the virus responsible for the COVID-19 pandemic. WHO IS THIS TEST FOR? COVID-19 ANTIGEN SELF-TEST ...
PRIMA Lab
Result display time: 3 min - 7 min
Specificity: 99.6 %
Sensitivity: 88.4 %
... errors Sealed test cassette - Easy to use and discard Internal controls included - Confidence in results due to confirmation of test strip functional integrity Room temperature storage - No need for ...
Alfa Scientific Designs
Result display time: 15 min - 20 min
... (SARS-CoV-2) N protein antigen in human nasal swabs Performance and features 02 Simple Individual test, easy to carry Rapid test kit, easy to perform 03 Convenient Visual ...
Aikang Diagnostics
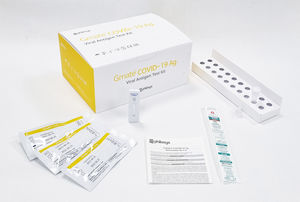
Result display time: 20 min
Gmate COVID-19 Ag kit is an in vitro diagnostic medical device that qualitatively detects antigens against COVID-19 in the nasopharyngeal smear of patients by immunochromatography. Specification Specimen volume ...
PHILOSYS Co., Ltd.

Result display time: 80 min
Sample volume: 0.012 ml
... fluorescent or above equipment Packing specification: 48 tests/box Detection limitation:100 copies/mL Sample processing method: no extraction Storage: cold chain transportation 220 tests / box size: ...


respiratory infection test kitMS01293
System for the presumptive identification and antimicrobial suceptibility test of the most common bacterial pathogens in Acute Respiratory Infections.
CPM SAS

Mytest S. pneumoniae Ag test is a lateral flow chromatographic immunoassay for the qualitative detection of Streptococcus pneumoniae Ag in human urine specimen. It is intended to be used as a screening test ...
Biofootprints Healthcare Pvt Ltd
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group






















Please specify:
Help us improve:
remaining