- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
Implantable venous port Celsite® Spinalsingle-lumensiliconepolyurethane
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Type of port
- venous
- Number of lumens
- single-lumen
- Material
- silicone, polyurethane, polyamide
Description
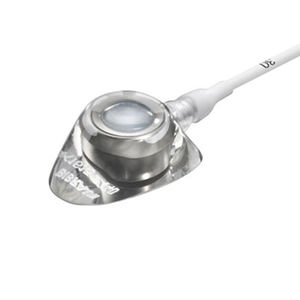
Celsite® Spinal
For spinal administration of pain relieving drugs
The Celsite® Spinal Access Ports Systems have an integrated 20μm titanium filter and are indicated for long-term spinal therapies, in particular pain relief.
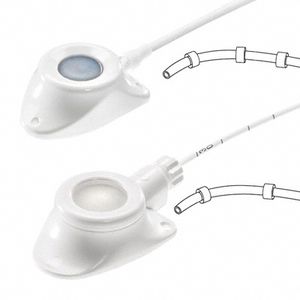
Celsite® Spinal offers epidural and spinal catheters for choice of implantation route.
Special Features
- Integrated 20μm titanium filter prevents the passage of particles
- Available with polyamide (epidural) and polyurethane (epidural and intrathecal) catheters
- Special lightweight access port with a plain polysulphone body
- Simple and secure screw clamp connection
General Features
- Anatomic design and lower profiled nose for simplified insertion and patient comfort
- High density silicone septum for reliable puncture and to ensure maximum port life
- Reliable radiopaque connection ring with anti-kink protection
- MRI compatible, Latex, DEHP and PVC free
Available Implantation Technique
- Intrathecal / epidural implantation technique
Additional Information
Every Celsite® Spinal reference contains 2 catheters:
- 1 multiperforated closed tip polyamide catheter (PA) – for epidural placement
- 1 open tip polyurethane catheter (PUR) with a teflon-coated guide wire – for epidural and Intrathecal placement
Catalogs
No catalogs are available for this product.
See all of Aesculap®‘s catalogsRelated Searches
- BBRAUN implantable port
- BBRAUN implantable single-lumen port
- BBRAUN implantable venous port
- BBRAUN implantable titanium port
- BBRAUN biopsy punch
- BBRAUN implantable polymer port
- BBRAUN bone biopsy punch
- Implantable silicone port
- Double-lumen implantable port
- Implantable intraperitoneal port
- Implantable polyurethane port
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.